TOPLINE:
Preserved ratio impaired spirometry (PRISm) was associated with increased odds of interstitial lung abnormalities (ILAs), and having both PRISm and ILAs was linked to a higher risk for mortality than having either PRISm or ILA alone or normal spirometry.
METHODOLOGY:
- PRISm has been recently defined as the concurrent reduction of both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) without airflow obstruction.
- Researchers aimed to compare the prevalence of ILAs in participants with PRISm with that in those with normal spirometry and to identify risk factors for ILAs in participants with PRISm by using data from a longitudinal cohort study of current or former smokers (COPDGene) from 2008 to 2011.
- They included 1262 participants with PRISm, defined as those with a postbronchodilator FEV1 < 80% predicted and FEV1/FVC ≥ 0.7, and 4494 participants with normal spirometry, defined as those with a postbronchodilator FEV1 ≥ 80% predicted and FEV1/FVC ≥ 0.7.
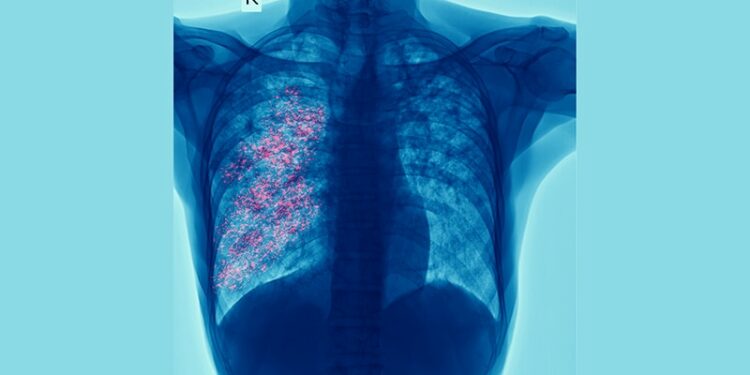
- ILAs were assessed radiologically using CT, and their progression was determined at the 5-year follow-up.
- The primary outcome was all-cause mortality assessed at the 5-year follow-up.
TAKEAWAY:
- PRISm was associated with higher odds of ILAs than normal spirometry (adjusted odds ratio, 1.74; 95% CI, 1.33-2.27; P < .001).
- Among participants with PRISm, having ILAs was independently associated with a higher risk for all-cause mortality than not having ILAs (adjusted hazard ratio [aHR], 2.58; 95% CI, 1.49-4.45).
- Using participants with normal spirometry without ILAs as the reference group, those with both PRISm and ILAs had a higher risk for mortality than those with either PRISm or ILAs alone (aHR, 4.5; 95% CI, 2.66-7.61).
- Among participants with PRISm, having ILAs was associated with older age, lower exercise capacity and FVC, a longer duration of smoking, and increased airway wall thickness.
IN PRACTICE:
“Overall, [the study] findings suggest that there is a subgroup of participants with PRISm who instead of following a pre-COPD [pre–chronic obstructive pulmonary disease] pathway, may instead be developing early ILD [interstitial lung disease], and may benefit from evaluation for ILA,” the authors wrote.
“Efforts should be made to increase smoking cessation given that higher duration and pack-years smoking are significantly associated with ILA in PRISm,” they added.
SOURCE:
This study was led by Sean Kalra, MD, Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston. It was published online on February 11, 2025, in CHEST.
LIMITATIONS:
Participants with PRISm and ILAs could not be subclassified on the basis of the cause of death, longitudinal spirometric transitions, or the progression of ILAs due to small sample sizes at follow-up. This study used FEV1 80% predicted rather than the lower limit of normal for maintaining consistency with the prior studies in COPDGene. Full pulmonary function tests were not included, which made it difficult to evaluate true restriction in the context of ILAs.
DISCLOSURES:
This study and several authors were supported by grants from the National Institutes of Health. COPDGene was supported by the COPD Foundation and grants from the National Heart, Lung, and Blood Institute. Two authors reported having financial ties with Bayer, AstraZeneca, and Northpond Laboratories.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/lung-abnormalities-prism-signal-higher-risk-death-2025a10007df?src=rss
Author :
Publish date : 2025-03-27 12:41:00
Copyright for syndicated content belongs to the linked Source.