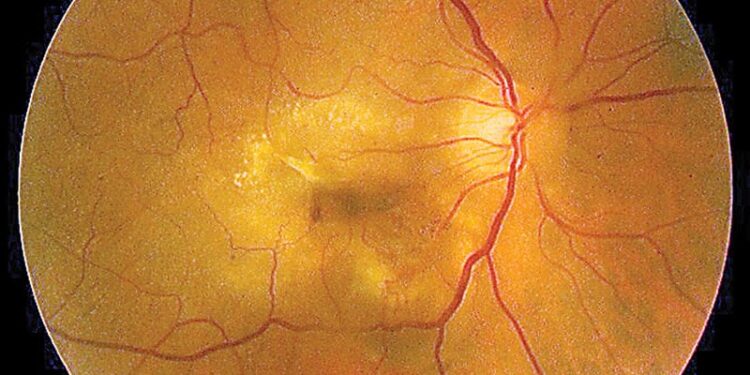
The sponsor of two phase 3 clinical trials of an add-on agent to treat age-related macular degeneration has decided to halt both trials after they showed the add-on treatment did not outperform either of the anti–vascular endothelial growth factor agents it was designed to boost when the medications were used alone.
The studies of sozinibercept 2 mg (Opthea), also known as OPT-302, failed to meet their primary endpoint of improvement in best corrected visual acuity (BCVA) after a year of treatment when compared with the singular agents. One trial evaluated sozinibercept in combination with aflibercept 2 mg (Eylea), the other in combination with ranibizumab 0.5 mg (Lucentis). The comparator in both trials was aflibercept or ranibizumab used alone.
Vascular endothelial growth factor is responsible for the growth of new blood vessels in neovascular age-related macular degeneration. Aflibercept targets VEGF-A and -B along with placental growth factor; ranibizumab targets VEGF-A. Sozinibercept acts by blocking VEGF-C and -D, according to Opthea.

“The hope was that by blocking VEGF-A, -C, and -D, we would see improved efficacy above and beyond our current VEGF-A blockade, especially given that there are a subset of patients who have a suboptimal response,” Jason Hsu, MD, codirector of Retina Research at Wills Eye Hospital in Philadelphia, told Medscape Medical News.
COAST and ShORe Trials
The aflibercept trial, known as COAST, evaluated intravitreal sozinibercept every 4 or 8 weeks in combination with intravitreal aflibercept administered every 8 weeks after three loading doses delivered 4 weeks apart, comparing with the use of aflibercept only every 8 weeks. The ranibizumab trial, known as ShORe, compared sozinibercept every 4 or 8 weeks in combination with ranibizumab every 4 weeks vs ranibizumab only.
After Opthea announced results last week of the failed COAST trial, the company decided to speed up the readout of topline data from the ShORe trial, which also failed to show the combination treatment with sozinibercept was superior to ranibizumab alone.
In COAST, patients receiving sozinibercept combination therapy with a dosing regimen of every 4 weeks or every 8 weeks had an average change in BCVA of 13.5 and 12.8 letters from baseline to week 52, respectively, vs 13.7 letters with aflibercept only (P = .86 and .42, respectively), the company reported.
In ShORe, patients who received sozinibercept combination therapy had an average improvement of 13.3 letters with treatment every 4 weeks or 12.6 letters with treatment after 8 weeks after a year of treatment, whereas those on ranibizumab only experienced an average improvement of 14.3 letters (P = .32 and .09, respectively), the company said.
“We are disappointed that COAST and ShORe did not demonstrate the improvements in vision with sozinibercept combination therapy compared to standard of care that we had hoped for,” Frederic Guerard, PharmD, CEO of Opthea, said in a press release.
In the press release, Opthea stated it must sort out a number of obligations to regulators and investors. “In light of these matters, there remains material uncertainty as to Opthea’s ability to continue as a going concern,” the company stated.
Hsu noted the phase 2b study of sozinibercept in combination with ranibizumab “looked promising,” and demonstrated an improvement in BCVA of more than three letters over ranibizumab alone, and gains of more than six letters in patients with occult choroidal neovascularization.
“Therefore, many of us were looking forward to the results of COAST and ShORe,” he said.
The phase 3 trials included a dosing regimen of sozinibercept injections every 8 weeks along with the standard dosing of every 4 weeks used in the phase 2b trial.
“In the published subgroup analysis of the phase 2b trial by lesion type, OPT-302 demonstrated the greatest benefit in occult lesions, so it will be important to see what proportion of patients in the phase 3 trials had occult lesions and if these proportions were similar to the phase 2b trial,” he said. “If there ended up being a higher proportion of minimally classic lesions in the phase 3 trials, for example, that may have contributed to the apparent negative study results. In retrospect, it might have been better to specifically focus on recruiting a high proportion of occult choroidal neovascularization.”
Adding a second agent to the standard anti–VEGF-A regiment in neovascular age-related macular degeneration has been “a high bar,” Hsu said. He cited the previously published CAPELLA phase 2 trial of an anti–platelet-derived growth factor receptor beta (rinucumab) combined with aflibercept to treat the neovascular form of the condition, which failed to show any benefit of the combined treatment.
The bispecific antibody faricimab (Vabysmo), which blocks both VEGF-A and angiopoietin-2, is one combination therapy that has demonstrated durability in treating neovascular age-related macular degeneration, Hsu said.
“I think there will be other combination therapies that may also prove fruitful in the future,” he said. “For instance, blocking other inflammatory mediators in combination with VEGF-A may prove to yield better results. I’m still optimistic.”
The phase 3 and phase 2b studies were funded by Opthea. Hsu disclosed his group has received grant support as a clinical trial center for Opthea, Genentech/Roche and Regeneron Pharmaceuticals.
Richard Mark Kirkner is a medical journalist based in Philadelphia.
Source link : https://www.medscape.com/viewarticle/sponsor-pulls-plug-trials-add-amd-treatment-2025a100081a?src=rss
Author :
Publish date : 2025-04-03 10:40:00
Copyright for syndicated content belongs to the linked Source.