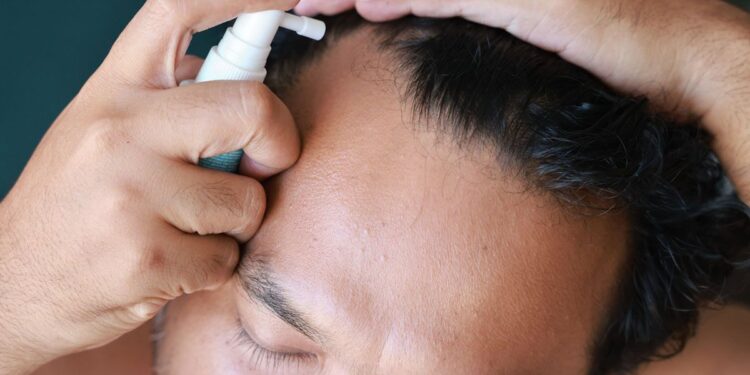
- The FDA has issued a warning about adverse effects related to a popular hair loss product available through telehealth platforms like Hims & Hers and Ro.
- Males have reported side effects of topical finasteride, including depression, dizziness, and low libido.
- Many consumers reported they were unaware or misinformed about the potential for serious side effects.
The Food and Drug Administration (FDA) issued a public warning about compounded topical versions of a popular hair loss drug sold through telehealth platforms.
However, the agency has not approved any topical formulations of finasteride. Despite this, such products are commonly marketed by direct-to-consumer telehealth companies, including Hims & Hers and Ro.
These products are “potentially putting consumers at risk,” the FDA cautioned in its statement.
According to the agency, users of compounded topical finasteride reported side effects similar to those associated with the oral form of the drug, including depression, fatigue, insomnia, and decreased libido.
“Some consumers expressed they became very depressed, suffering with pain and their lives were ruined because of these symptoms,” the FDA noted in its warning.
In many cases, consumers said they were not properly informed about these risks, or were even assured by prescribers that topical use carried no risk of side effects. Unlike pharmaceutical companies, telehealth platforms are not held to the same regulatory standards and are not required to disclose potential side effects in their advertising.
Anthony Oro, MD, PhD, Eugene and Gloria Bauer Professor of Dermatology at Stanford Medicine, told Healthline that the reported side effects were consistent with expectations.
“There’s no surprise about the adverse effects, because those are the same that have been seen with the oral medicine,” Oro said. “The disconnect is that people may think because it’s topical that it’s safe and can be used widely at different concentrations without any problems.”
Healthline contacted Hims & Hers and Ro to comment on the FDA warning but did not receive a response.
The FDA alert underscores broader concerns about compounded medications, which the agency does not review or approve. As telehealth services make these products more accessible, experts warn that patients may be unaware of the potential risks.
The FDA warning is the latest high-profile incident involving compounded finasteride.
In March, the Wall Street Journal published an investigation detailing adverse effects reported by men who had purchased the drug through telehealth platforms.
Among those interviewed was U.S. Army Sgt. Mark Millich, 26, who said he experienced symptoms such as anxiety, dizziness, and slurred speech. He also reported severe sexual side effects, including reduced libido and genital shrinkage.
- erectile dysfunction
- reduced sex drive
- ejaculation disorder
- depression
- dizziness
“Most of the side effects the FDA is seeing for topicals correspond to those that are known from the approved oral formulation. It’s not that there’s a new adverse side effect that are being reported,” said Oro.
Some males also reported long-lasting sexual and psychological effects long after ceasing to take the drug, a condition referred to as
Additionally, the FDA notes that there may be additional risks to using a topical form of the drug, including:
- localized irritation
- erythema
- dryness
- stinging or burning
The FDA also warns that since topical finasteride is applied directly to the skin, it may inadvertently transfer to others through contact, an issue with potentially serious consequences.
The drug is contraindicated during pregnancy due to its potential to cause abnormalities in a developing male fetus.
Compounded drugs are not FDA-approved, and there is often little data to determine safety.
While both minoxidil and finasteride are individually FDA approved, the safety profile of a compounded drug containing both is unclear.
While the reported symptoms from the FDA’s warning align with known side effects of oral finasteride, the key concern is that consumers either were not informed of the risks or were led to believe they didn’t apply to topical versions of finasteride.
“A lot of people have a misconception that because it’s topical it’s not going to be absorbed systemically,” said Oro.
Such a misconception appears to be propagated by telehealth companies.
On Ro’s website under side effects related to topical finasteride, the company states, “Because topical finasteride does not enter the bloodstream in the same way or quantity oral finasteride does, there’s reason to assume that topical finasteride could come with a lower risk of systemic effects, including sexual side effects.”
Hims describes the potential side effects of topical finasteride as “minimal”, such as skin irritation and itching, typically localized to where the medicine is applied. The company also cites a study supporting the claim that “topical finasteride also comes with a lower risk of sexual side effects than oral finasteride due to its localized use.”
According to the
With the proliferation of telehealth platforms and expanded access to compounded medications, Oro told Healthline that nothing can replace the doctor-patient relationship required for informed decision-making.
“You need to have someone who understands the medication and the patient — when it is appropriate to use and when you need to stop,” said Oro. “There’s a trend now where you have access to medication without a long-term relationship between the patient and the provider, and we are seeing some of the ill effects of that now.”
Source link : https://www.healthline.com/health-news/fda-warning-hair-loss-treatment-topical-finasteride
Author :
Publish date : 2025-04-25 11:56:45
Copyright for syndicated content belongs to the linked Source.