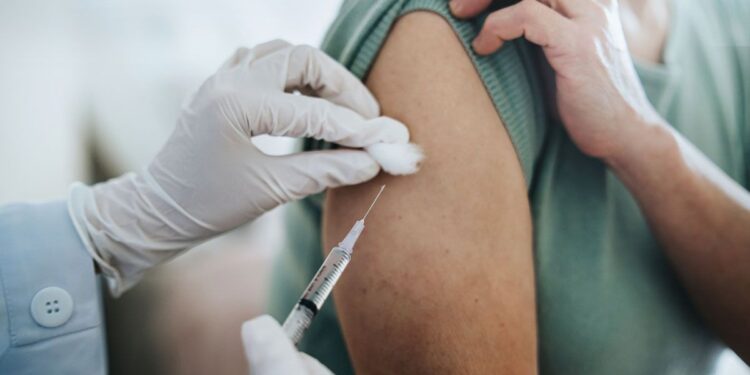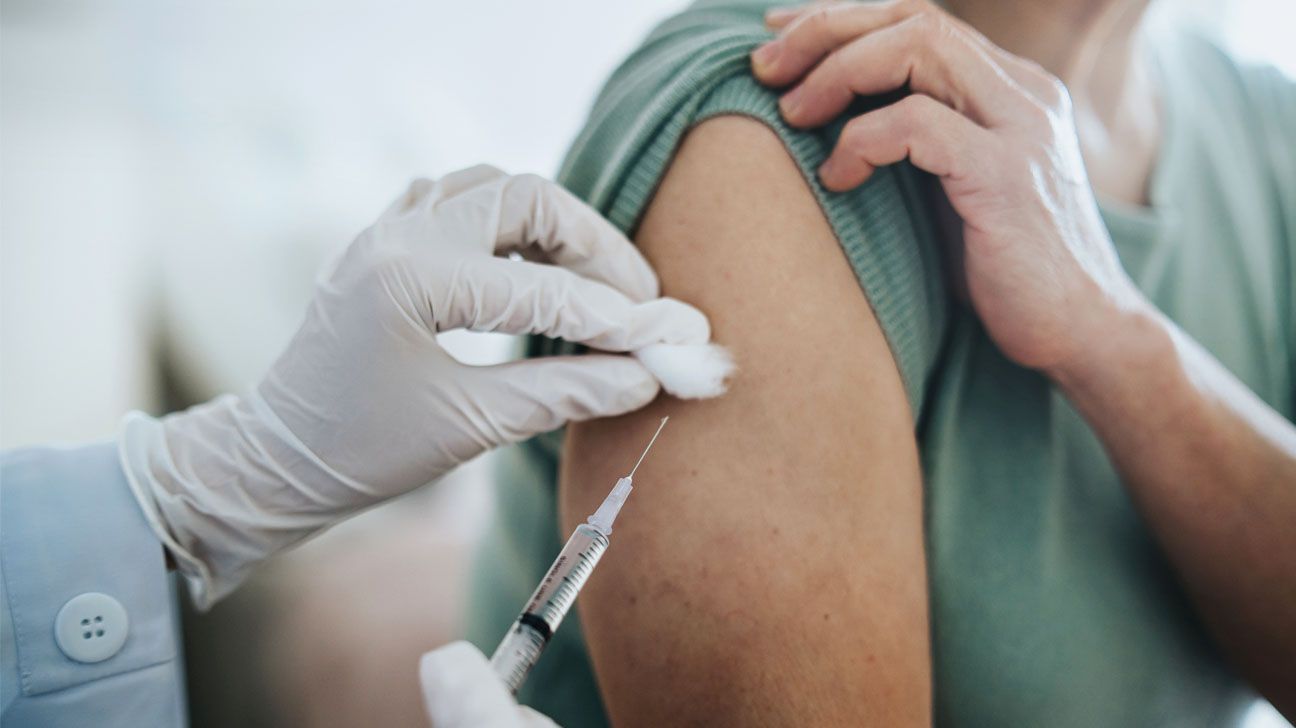
- The FDA has approved lenacapvir, a twice-yearly injection to prevent HIV infection that could improve adherence rates compared to other PrEP medications.
- The drug, which will be sold under the brand name Yeztugo, showed near-total effectiveness in clinical trials with minimal side effects.
- Experts are excited about using lenacapvir for HIV prevention, but remain cautious about the drug’s affordability and accessibility.
A drug used to treat some HIV infections can now be used to help prevent HIV, the virus that causes AIDS.
On June 18, lenacapavir, a twice-yearly injection from Gilead Sciences, gained approval from the Food and Drug Administration (FDA) for HIV prevention in adults and adolescents.
The drug, which will be sold under the brand name Yeztugo, significantly reduced the risk of HIV infection in two phase 3 clinical trials that were funded by Gilead.
The PURPOSE 1 and PURPOSE 2 trials, which were highly inclusive by design, showed that 99.9% of participants who received lenacapavir remained HIV negative. The side effects were also minimal, mostly pain or swelling at the injection site.
PrEP treatments have been used in the United States to prevent HIV since 2012. These therapies generally involve daily oral medication (Truvada) or injections every two months (Apretude).
Now, a biannual injection option offers an effective and appealing alternative for HIV prevention with less commitment. Yeztugo is the first and only twice-yearly option for HIV prevention available in the United States for those who may need or want PrEP.
The FDA approval of this prevention therapy follows recently announced federal cuts to funding for an HIV vaccine program, marking a silver lining amid uncertainty in HIV research.
“Yeztugo could be the transformative PrEP option we’ve been waiting for — offering the potential to boost PrEP uptake and persistence and adding a powerful new tool in our mission to end the HIV epidemic,” Carlos del Rio, MD, Distinguished Professor of Medicine in the Division of Infectious Diseases at Emory University School of Medicine and co-director of the Emory Center for AIDS Research in Atlanta, said in a news release.
“A twice-yearly injection could greatly address key barriers like adherence and stigma, which individuals on more frequent PrEP dosing regimens, especially daily oral PrEP, can face. We also know that, in research, many people who need or want PrEP preferred less frequent dosing,” del Rio continued.
Despite advancements in HIV treatment and prevention, in 2023,
A recent CDC analysis shows that up to 2.5 million U.S. adults need PrEP to prevent HIV infection. However,
PrEP therapies are widely available in the United States, but the medication
Experts say having a highly effective, twice-yearly PrEP option for HIV prevention could improve access for people with and without insurance and bolster adherence rates.
“Providers are excited about the approval of long-acting lenacapavir for HIV prevention since this once-every-six-month injection has been shown to have high efficacy in preventing HIV in both women and men in two large trials,” said Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco. Gandhi wasn’t involved in the clinical trials.
“Data from our clinic in San Francisco, which serves low income people with or at risk of HIV, and others have shown that long-acting PrEP works well for people living with HIV who have high rates of concomitant challenges such as housing insecurity and substance use where it can be difficult to take a daily oral pill for PrEP,” Gandhi told Healthline.
Gandhi further noted that higher retention rates have been observed among those on long-acting PrEP with another long-acting agent, Apretude (cabotegravir), compared to oral PrEP alone.
She anticipates similar improved adherence rates for those on long-acting Yeztugo (lenacapavir), both for those who have difficulty taking an oral pill or those who might prefer the convenience of a twice-yearly injection.
Within the United States, men who have sex with men, as well as heterosexual individuals with multiple partners, are considered high risk for HIV transmission.
But the need for accessible HIV prevention therapies like PrEP spans the globe.
According to Gilead, regulators around the world are reviewing Yeztugo for HIV prevention. For now, the United States is the only country to approve the drug.
“It’s very exciting and we have a great deal of confidence in it,” William Schaffner, MD, professor of preventive medicine and infectious diseases in the Department of Health Policy at Vanderbilt University Medical Center in Nashville, said of the drug. Schaffner wasn’t involved in the clinical trials.
“It could be a game-changer,” he told Healthline. “Short of a vaccine, this is a way to efficiently prevent HIV infection in large populations that are at high risk, not only in the developed world, but potentially in the developing world also. If we have the determination, followed by the allocation of resources to reach out to populations at high risk, we can make this available,” Schaffer noted.
It’s unclear when Yeztugo will become available to the general U.S. population and how much it might cost out of pocket, particularly for those without health insurance.
“I don’t know how quickly the company can make this available and educate practitioners about its utility, but certainly both in the public health sector — and we’ll see where the resources extend to — and in private medical circumstances, we may have to wait a little while before medical insurance programs include Yeztugo in their benefits packages,” Schaffer said.
Gandhi expressed concern over the drug’s high cost and whether it would be covered by large health insurance companies. Gilead said lenacapavir could cost around $28,218 annually per person, The New York Times reported.
“Although the total cost per year is not that different than the total cost of long-acting [Apretude] cabotegravir for PrEP in a year, these are days of austerity in healthcare, including the concerning possibility of a $700 billion cut to Medicaid which will be determined in an upcoming Senate vote, leaving the health insurance program for low-income individuals in the U.S. severely underfunded. I urge the company to reconsider their price to improve access for Americans,” Gandhi said.
Source link : https://www.healthline.com/health-news/fda-approves-twice-yearly-injection-hiv-prevention
Author :
Publish date : 2025-06-23 12:50:36
Copyright for syndicated content belongs to the linked Source.