TOPLINE:
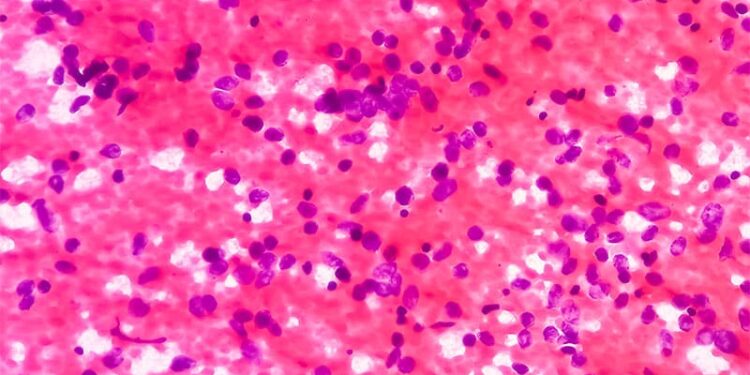
In a multicentre study, lurbinectedin demonstrated a favourable safety profile and consistent efficacy and may be considered for compassionate use in patients with extensive-stage small cell lung cancer (ES-SCLC). However, poor performance status, a chemotherapy-free interval of less than 90 days, and the presence of brain or liver metastases may have negatively affected overall survival (OS).
METHODOLOGY:
- This multicentric, international cohort included 238 adult patients with ES-SCLC (median age, 65 years) who received lurbinectedin intravenously at 3.2 mg/m2 every 3 weeks as second- or further-line treatment between November 2019 and September 2024.
- The primary objective was to assess the effectiveness of lurbinectedin with regard to objective response rate, disease control rate, duration of response, progression-free survival (PFS), and OS and its safety profile.
- The median follow-up duration was 5.53 months.
TAKEAWAY:
- Overall, 37% of patients received lurbinectedin as second-line therapy, 45% received it as third-line therapy, and 18% received it as further-line therapy. The objective response rate was 23.1%, and the disease control rate was 45.4%.
- The median PFS was 2.2 months, and the median OS was 5.4 months. The 6-month PFS and OS rates were 12.2% and 42.4%, respectively.
- Patients with a chemotherapy-free interval of 90 days or more showed significantly longer PFS (3.1 vs 1.8 months; hazard ratio [HR], 0.46; P < .001) and OS (6.8 vs 4.5 months; HR, 0.56; P = .006) than chemoresistant patients.
- Eastern Cooperative Oncology Group performance status of two or more at treatment start and the presence of brain or liver metastases were associated with worse outcomes.
- Treatment-related adverse events (AEs) of any grade were recorded in 92% of patients, with 29% of patients experiencing at least one grade 3-4 toxicity and the most frequent being neutropenia that occurred in 22% of patients.
IN PRACTICE:
“Our study provides valuable real-world insights into the effectiveness and safety of lurbinectedin as compassionate use treatment for ES-SCLC, supporting its use with outcomes consistent with those observed in clinical trials and other real-world studies. However, the outcomes for patients with poor PS [performance status] at lurbinectedin start, a CFI [chemotherapy-free interval] of less than 90 days, and brain or liver metastases remain suboptimal and this should be carefully considered when making treatment decisions,” the authors of the study wrote.
SOURCE:
This study was led by Daniela Scattolin, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy. It was published online on July 02, 2025, in the European Journal of Cancer.
LIMITATIONS:
This study was limited by its retrospective design, the small number of participating centres, and imbalanced cohort sizes between countries. Researchers noted heterogeneity in baseline patient characteristics, treatment management strategies, and tumour assessment protocols. Additionally, differences in national regulations regarding chemoimmunotherapy use could have introduced bias. The retrospective nature of data collection may have resulted in underreporting of AEs.
DISCLOSURES:
This study did not receive any specific funding. Several authors reported receiving speaker/consultant fees and having other ties with various sources. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/lurbinectedin-shows-modest-efficacy-and-safety-extensive-2025a1000ias?src=rss
Author :
Publish date : 2025-07-14 12:00:00
Copyright for syndicated content belongs to the linked Source.