At its July 2025 meeting, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorization for sebetralstat (Ekterly, KalVista Pharmaceuticals) for symptomatic treatment of acute attacks of hereditary angioedema in adults and adolescents aged 12 years or older.
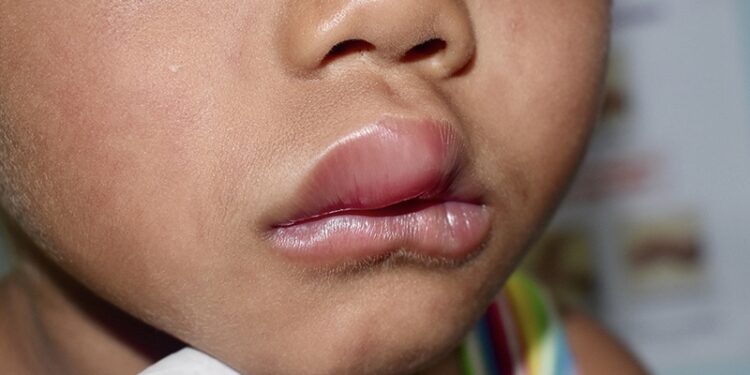
Hereditary angioedema is a rare, chronic, and potentially life-threatening genetic condition caused by deficiency or dysfunction of C1 esterase inhibitor, leading to excessive bradykinin and unpredictable swelling episodes in the face, abdomen, limbs, and airways.
Sebetralstat is a selective oral plasma kallikrein inhibitor designed for administration at the earliest sign of an angioedema attack. It blocks the overactivation of the kallikrein-kinin system, preventing the formation of bradykinin, a key mediator of swelling in the condition.
If approved by the European Commission, the drug will be the first oral on-demand treatment for hereditary angioedema in the European Union.
The CHMP opinion was based on efficacy and safety data from a phase 3 KONFIDENT trial, which randomized 136 patients with hereditary angioedema. Findings showed that sebetralstat led to significantly faster symptom relief and reduction in attack severity and attack resolution than placebo. It was also well-tolerated with a safety profile similar to placebo.
Ekterly will be available in 300-mg film-coated tablets. The most common side effect reported is headache.
One of the key advantages of the drug is its oral route of administration, offering patients a more convenient alternative to injectable therapies. This may significantly reduce the time between an angioedema attack onset and treatment administration.
Ekterly was granted orphan medicine status during its development due to its targeted use in a rare, life-threatening condition. The EMA noted that the drug would have been unlikely to be developed without such incentives and is now reviewing the available data to determine whether the orphan designation should be maintained.
Source link : https://www.medscape.com/viewarticle/ema-backs-first-oral-treatment-hereditary-angioedema-2025a1000jvq?src=rss
Author :
Publish date : 2025-07-28 15:21:00
Copyright for syndicated content belongs to the linked Source.