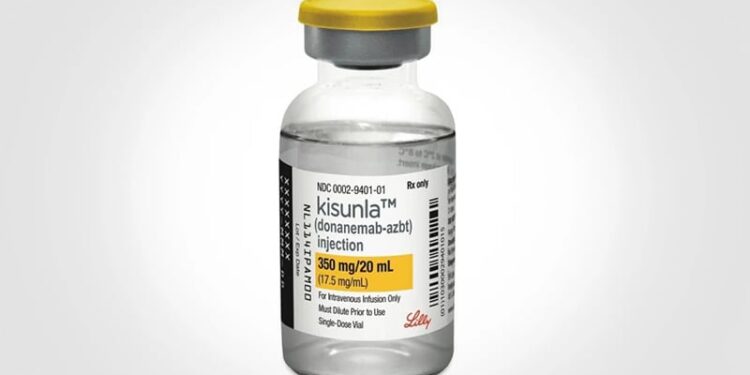
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Kisunla (donanemab, Eli Lilly Nederland) for treating early symptomatic Alzheimer’s disease in adults who are apolipoprotein E epsilon 4 noncarriers or heterozygotes.
This recommendation overturns the previous negative opinion issued earlier this year by the committee, which concluded that the benefits of this medicine did not outweigh the risks of potentially fatal events associated with amyloid-related imaging abnormalities (ARIA), including brain swelling and the potential for bleeding.
Kisunla’s active substance is donanemab, a monoclonal antibody that targets and clears amyloid-beta plaques in the brain, a key feature of Alzheimer’s disease. By reducing plaque burden, the therapy aims to slow cognitive decline in the early symptomatic stage of the disease.
Kisunla will be available as a 350 mg concentrate for solution for intravenous infusion.
The CHMP’s positive opinion was based on data from the phase 3 TRAILBLAZER-ALZ 2 trial, which enrolled 1736 participants aged 60-85 years with early symptomatic Alzheimer’s disease (mild cognitive impairment/mild dementia) with amyloid and low/medium or high tau pathology, as confirmed by PET imaging. Participants were randomized to receive either donanemab (n = 860) or placebo (n = 876) intravenously every 4 weeks for 72 weeks.
Donanemab significantly improved integrated Alzheimer’s Disease Rating Scale (iADRS) and Clinical Dementia Rating Scale Sum of Boxes scores compared with placebo. In the low/medium tau population, the change in iADRS at 76 weeks was -6.02 for donanemab vs -9.27 for placebo.
The most common side effects associated with donanemab are ARIA and headache. Other reported side effects include infusion-related reactions.
The European Commission will now review the CHMP’s recommendation to make a final decision on Kisunla’s marketing authorization in the European Union. If approved, treatment should be initiated by clinicians experienced in Alzheimer’s management, with access to MRI, and under the supervision of a multidisciplinary team trained in managing ARIA and infusion-related reactions.
Detailed usage recommendations will be outlined in the product’s summary of characteristics, which will be published on the EMA website after marketing authorization.
Source link : https://www.medscape.com/viewarticle/ema-reverses-previous-refusal-kisunla-alzheimers-2025a1000k8x?src=rss
Author :
Publish date : 2025-07-30 14:32:00
Copyright for syndicated content belongs to the linked Source.