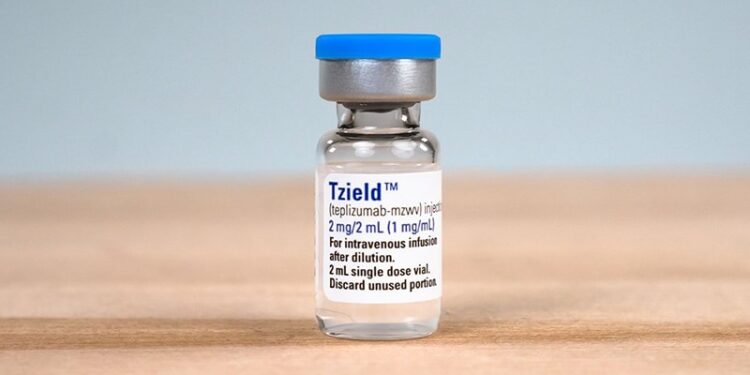
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved teplizumab (Tzield, Aventis Pharma Limited) as the UK’s first immunotherapy for type 1 diabetes (T1D).
The drug is licensed for adults and children aged 8 years and older with stage 2 T1D to delay progression to stage 3.
Teplizumab was approved in the US in 2022 by the Food and Drug Administration after a clinical trial showed it could delay the onset of symptomatic T1D by an average of 3 years in people at high risk who were in the early, symptomless stages.
Around 8% of people with diabetes in the UK have T1D – about 400,000 people. It is the most common type of diabetes in children and young people. The UK has one of the highest rates in Western Europe, with an estimated incidence of 193.8 per 100,000 in younger age groups.
Delaying Progression Protects Against Complications
Once stage 3 T1D develops, people usually begin to experience blood sugar problems and often require lifelong insulin treatment. Delaying progression allows patients to remain free from insulin treatment, carbohydrate counting, and hypoglycaemia episodes for longer. It also helps maintain healthy blood glucose levels, reducing long-term complication risks.
A later age at diagnosis may mean patients are better able to manage the condition, potentially avoiding childhood onset altogether.
The MHRA said the approval came through its international recognition procedure, which allows the agency to draw on the expertise and decision-making of trusted regulatory partners.
‘New Era’ for T1D Treatment
Teplizumab tackles the root cause of T1D – the immune system attack on insulin-making pancreatic beta cells. Slowing this attack can protect beta cell function.
Diabetes UK described the approval as “a milestone moment and the beginning of a new era for T1D treatment”. The charity said it would continue to lobby the National Institute for Health and Care Excellence (NICE) to recommend the drug for routine NHS use in England.
NICE is expected to revisit its draft decision not to recommend teplizumab when its evaluation committee meets in October.
Although licensed only for early-stage T1D, Diabetes UK said that clinical trials suggest it may also benefit newly diagnosed patients by protecting the roughly 20% of beta cells that remain, potentially making blood sugar management easier and reducing long-term risks.
While T1D cannot yet be prevented, the charity said that immunotherapies, combined with screening for diabetes autoantibodies to identify people in the early stages, could offer a way to hold off and delay its full development for as long as possible.
Call for Screening
Dr Elizabeth Robertson, director of research and clinical at Diabetes UK, said the next steps were critical.
“To ensure teplizumab reaches everyone who could benefit, we need it to be made available on the NHS, and the rollout of a screening programme to identify those with early-stage T1D,” she said.
Researchers hope future advances could extend the delay — potentially for life — or lead to a cure for people already living with T1D.
Treatment and Safety
Teplizumab is given as a daily intravenous infusion for 14 days in a hospital setting.
The most common side effects are rash, leukopenia with an increased risk of infections, and headache. The full list will be published on the MHRA website within 7 days.
The MHRA said it would closely monitor the drug’s safety and urged clinicians and patients to report suspected side effects via its Yellow Card scheme.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
Source link : https://www.medscape.com/viewarticle/mhra-approves-first-t1d-immunotherapy-uk-2025a1000lnn?src=rss
Author :
Publish date : 2025-08-15 10:54:00
Copyright for syndicated content belongs to the linked Source.