TOPLINE:
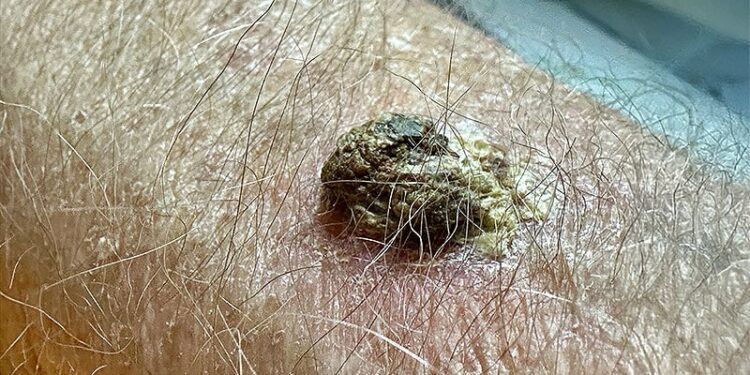
Adjuvant cemiplimab therapy reduced the risk for disease recurrence or death by 68% compared with placebo in patients with high-risk cutaneous squamous cell carcinoma after surgery and radiotherapy. The 24-month disease-free survival rate was 87.1% vs 64.1% with cemiplimab vs placebo.
METHODOLOGY:
- The primary treatment, surgery with curative intent, achieves a cure in approximately 95% of patients. Previous trials helped identify patient subpopulations at highest risk for recurrence, but the benefit of systemic adjuvant therapy options has not been well established in clinical trials.
- A phase 3, randomized trial enrolled 415 patients with local or regional cutaneous squamous cell carcinoma who completed surgery and postoperative radiotherapy, with 209 receiving cemiplimab and 206 receiving placebo.
- Participants received cemiplimab (350 mg) or placebo intravenously every 3 weeks for 12 weeks, followed by 700 mg every 6 weeks for up to 36 weeks (≤ 48 weeks total).
- The primary endpoint was disease-free survival, with secondary endpoints including freedom from locoregional recurrence, freedom from distant recurrence, and safety.
- Researchers recruited patients from 107 sites across 16 countries, with a median follow-up of 24 months.
TAKEAWAY:
- Cemiplimab vs placebo demonstrated superior disease-free survival with 24 vs 65 events (hazard ratio [HR], 0.32; 95% CI, 0.20-0.51; P < .001).
- Treatment with cemiplimab led to lower risks for locoregional recurrence (9 vs 40 events; HR, 0.20; 95% CI, 0.09-0.40) and distant recurrence (10 vs 26 events; HR, 0.35; 95% CI, 0.17-0.72).
- Adverse events of grade 3 or higher occurred in 23.9% of cemiplimab patients vs 14.2% of placebo patients, with discontinuation rates of 9.8% and 1.5%, respectively.
- Overall survival at 2 years was 94.8% (95% CI, 89.6-97.4) with cemiplimab vs 92.3% (95% CI, 86.5-95.7) with placebo (HR, 0.86; 95% CI, 0.39-1.90).
IN PRACTICE:
“Adjuvant cemiplimab therapy led to longer disease-free survival than placebo among patients at high risk for recurrence of cutaneous squamous cell carcinoma. Because anti-PD-1 therapy provides durable responses in less than 50% of patients in the context of advanced cutaneous squamous cell carcinoma, the ability of adjuvant cemiplimab to reduce the risk of recurrence of cutaneous squamous cell carcinoma is clinically meaningful for patients at high risk for recurrence,” wrote the authors of the study.
SOURCE:
The study was led by Danny Rischin, MD, Peter MacCallum Cancer Centre in Melbourne, Australia. It was published online on May 31 in The New England Journal of Medicine.
LIMITATIONS:
According to the authors, the trial was not designed to formally investigate differences in efficacy and safety between the two dose regimens. Additionally, at the time of primary analysis, with only 25 deaths observed, a convincing benefit regarding overall survival has not been demonstrated, although follow-up is ongoing.
DISCLOSURES:
The study was supported by Regeneron Pharmaceuticals and Sanofi. Rischin received support from a National Health and Medical Research Council Investigator Grant. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/cemiplimab-extends-disease-free-time-skin-cancer-post-op-2025a1000lu7?src=rss
Author :
Publish date : 2025-08-19 06:01:00
Copyright for syndicated content belongs to the linked Source.