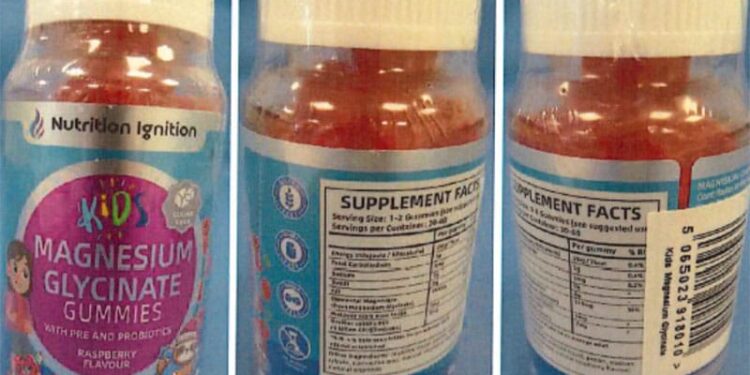
The Medicines and Healthcare products Regulatory Agency (MHRA) has warned that a children’s magnesium supplement contains undeclared melatonin, a prescription-only medicine in the UK.
The alert applies to Nutrition Ignition Kids Magnesium Glycinate Gummies. Parents and caregivers have been urged to stop using the product immediately.
The gummies, marketed and sold online as food supplements, should have been regulated as medicines and hold a UK licence to be legally sold, the MHRA said. The agency has worked with online retailers to remove the product from sale.
Prescription-Level Doses Detected
Tests on two batches identified between 1.5 mg and 1.7 mg of melatonin per gummy. The packaging recommended one or two gummies daily, depending on the age of the child. The labelling suggested that the product supports “calm, focus, and digestion” for children aged 4 years and over.
The presence of melatonin is not listed on the packaging, the MHRA noted.
Medical Use of Melatonin
Melatonin is a hormone produced naturally in the brain in cyclical fashion, with low circulating levels during the day and a peak close to the natural sleep time. It helps regulate sleep by promoting drowsiness and supporting the ability to fall asleep. Supplementary melatonin is chemically similar to the natural hormone.
In the UK, melatonin is available only as a prescription medicine. It is authorised for children aged 6 years or older, and for adults, when other sleep management approaches have not been effective.
Indications include insomnia in children and adolescents (aged 6-17 years) with attention-deficit/hyperactivity disorder or delayed sleep-wake phase disorder, where sleep hygiene measures alone have not worked.
Some formulations are also licensed for single use as short-term sedation under medical supervision to facilitate electroencephalograms in children and adolescents aged 1-18 years. In adults, melatonin may be prescribed for the management of jet lag.
Side Effects and Safety
Taking too much melatonin can cause drowsiness, headache, dizziness, and nausea. However, the MHRA said that lasting harm is unlikely, even from ingesting melatonin at high levels, as the body typically clears the drug within 12 hours.
Occasional case reports have described exacerbation of an autoimmune disease in patients taking melatonin, and it is not recommended in patients with such conditions. The drug should also be used with caution in people with epilepsy.
The MHRA urged anyone with the gummies at home to store them in a tamper-proof container until they can be returned to a pharmacy for safe disposal.
Dr Alison Cave, the MHRA’s chief safety officer, said: “We advise any parent or caregiver to stop use of this product and safely dispose of it.”
Side effects should be reported to the MHRA Yellow Card scheme.
Source link : https://www.medscape.com/viewarticle/uk-regulator-warns-melatonin-childrens-gummies-2025a1000luw?src=rss
Author :
Publish date : 2025-08-19 11:18:00
Copyright for syndicated content belongs to the linked Source.