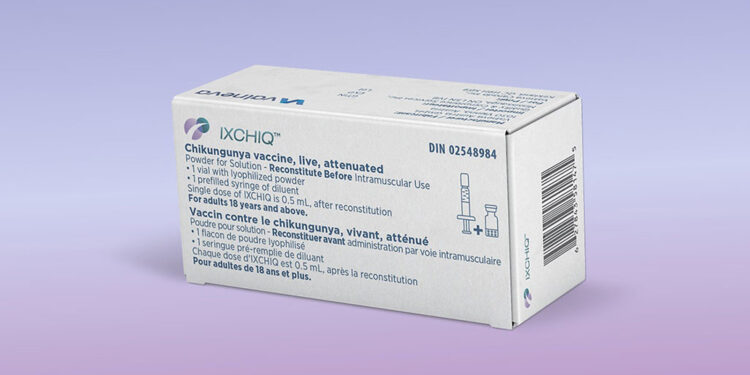
(MedPage Today) — Just 2 weeks after lifting a pause on the use of the chikungunya live-attenuated vaccine (Ixchiq) in people 60 years and older, the FDA suspended the vaccine’s license entirely over “serious” safety concerns.
(MedPage Today) — Just 2 weeks after lifting a pause on the use of the chikungunya live-attenuated vaccine (Ixchiq) in people 60 years and older, the FDA suspended the vaccine’s license entirely over “serious” safety concerns.
The Center for…
Source link : https://www.medpagetoday.com/publichealthpolicy/productalert/117140
Author :
Publish date : 2025-08-25 17:04:00
Copyright for syndicated content belongs to the linked Source.