Ian Burkhart was on holiday with friends in 2010 when his life changed forever. He dived into shallow water and broke his neck, leaving him paralysed from the shoulders down at the age of 19. “At that point, I was getting assistance with everything,” he says, “even being able to scratch an itch on my forehead.”
A few years later, Burkhart got an experimental brain implant that rerouted nerve impulses around his broken spinal cord to the muscles of his arm. It took time, but eventually he was able to use his hands and arms again – and even play the video game Guitar Hero. All in all, it worked pretty well.
Until, that is, it didn’t. Because that’s the thing about the implants we use to repair damaged or failing bodies: they don’t last forever. That goes for devices of all kinds, including the 3 million or so pacemakers inside people’s chests worldwide. Rigid, artificial hardware just doesn’t sit well with the soft and squishy human body; eventually, the immune system turns against these foreign materials.
Now, however, a new type of electronics is being developed in labs around the world that might just change all this. The vision is for implants made from materials that bend, flow and even grow alongside our nerves and muscles, helping them to sit more harmoniously inside us. Some are built from soft, tissue-like materials. Others go further and blend living cells with circuitry. Together, they are forging a new class of “living electronics” that aim to not only last much longer, but also to one day help rebuild the body’s lost connections.
We have known for centuries that electricity and biology are intimately linked. In the 18th century, Italian scientist Luigi Galvani provided the first concrete demonstration by making frog legs twitch with a jolt of charge. Today, we understand the human body runs on subtle electrical impulses that drive everything from heartbeats to wound healing.
Why implants fail
Modern implants tap into this circuitry. Pacemakers maintain the heart’s rhythm. Deep brain stimulators send electrical pulses into our brains to alleviate symptoms of Parkinson’s disease. Cochlear implants can stimulate nerves in the inner ear to restore hearing. Efforts are also under way to develop retinal implants that reinstate a person’s sight.
How well the body copes with these devices varies wildly. Pacemakers are usually tolerated well, lasting up to 15 years until their batteries need to be replaced, but deep brain stimulators generally need to be replaced every three to five years. Burkhart’s experimental brain implant was a record-breaker at the time, lasting an impressive seven years before it had to be removed.

One company has developed a thin microelectrode array that sits on the brain’s surface, sending it signals without damaging the delicate tissue.
Precision Neuroscience
These implants are all bedevilled by a common problem: the immune system. Most tissues are soft, and rigid electronics can cut through them like a blade, says Jia Liu at Harvard University. Over time, this causes tiny injuries. The immune system responds, inflammation follows and, eventually, scar tissue surrounds the implant, degrading its signal and sometimes forcing its removal. Our tissues constantly writhe around and can change in shape and density as we age, all of which compounds the problems. Stiff devices just can’t keep up, slipping out of place or tearing surrounding tissue, which triggers further immune attacks.
Making softer implants
One straightforward fix is to make these implants softer. Several companies have embraced this approach, including Elon Musk’s Neuralink, which makes a thread-like brain implant, and Precision Neuroscience, whose electrode arrays are encased in a soft, film-like polymer that rests on the brain’s surface.
But Liu’s lab is pushing the envelope further. It has created an electrode thread so soft that it is almost invisible in water, yet tough enough to survive implantation and the harsh chemicals used in manufacturing. Crucially, the device can pack in almost 100 times as many electrodes per thread as Neuralink’s, boosting the amount of data that can be recorded or transmitted. The probe causes minimal immune response, and Liu thinks that could allow it to remain effective for much longer than implants like Burkhart’s. His start-up, Axoft, recently completed a clinical trial demonstrating that the probes could be safely implanted in human brains and record neural information.
Biohybrid implants that grow
Still, softness isn’t the only route to successful implants. Early in her career, Rylie Green at Imperial College London was working on ideas similar to Liu’s. But, in around 2012, she watched a sci-fi show featuring organic space stations that grow arms to connect with docking spaceships. That got her thinking. “Why can’t our devices grow into the tissue?” she says. “Instead of sitting there and waiting for the body to respond, why can’t we have a more proactive device?”
She and her team have now developed a tiny probe designed to do just that. At its core is a small circular platinum electrode that is coated in a jelly-like hydrogel – a soft, flexible material that can absorb and store fluids, and that feels a lot like biological tissue. Green’s team seeds this with living neurons, which grow branches that connect with the host’s brain tissue, while staying controllable from the device’s electrode. Her group is developing implants to read brain activity or deliver deep stimulation for conditions such as epilepsy or Parkinson’s.
At the University of Cambridge, George Malliaras is taking a complementary path, having exchanged ideas with Green for years. His approach merges two cutting-edge biomedical fields: bioelectronics and therapies that treat diseases by using stem cells to repair or replace damaged tissues.
The strategy is much like a Trojan horse. The idea is to cloak the bioelectronics in living cells that can provide a biological camouflage. That way, the implant can not only evade the body’s immune systems, but also merge with existing tissues.
In a study published in 2023, Malliaras and his team created a flexible, film-like electrode and coated the end with a hydrogel seeded with stem cells. These cells were coaxed into forming muscle tissue and then the cell-coated end was sutured to the severed nerve ending of a rat’s forearm. The use of tissue meant the body wouldn’t reject the implant, says Malliaras, but it also prompted the rat’s neurons to grow into the implanted muscle cells. This biological bridge allowed the team to record electrical activity from the rat’s nerve at a resolution much higher than a standard electrode implant.
The group is now working on an implant that can bridge the severed nerve completely, not just recording electrical signals, but restoring movement to a paralysed limb. In the long run, Malliaras envisions versions that can transmit signals both ways – sending movement commands out and bringing sensation back in, whether to and from a real limb or a prosthesis. He says the device is about three to five years away from being tested in people.
But the benefits of biohybrid approaches may go deeper still. Researchers are already experimenting with transplanting stem cells into people with spinal cord injuries or neurodegenerative conditions. These cells can develop into many different types of nerve cells – but how they mature and wire up to other cells is heavily influenced by the body’s internal electrical signals, particularly during early development.
In adults, those guiding signals are mostly absent, says Malliaras. But biohybrid approaches could help to replicate these signals, and even speed up and control the regenerative process, he says. There is evidence that electrically stimulating stem cells can help guide how they differentiate, migrate and even function in the body.
“We’re using a very limited capability of electronics when we interface with cells today,” says Malliaras. “I can envision bioelectronics 2.0, where you implant the electrode not just to control an existing neuronal system, but using electrical stimulation to rebuild the network and then control it.”
That could open up the tantalising possibility of going beyond simply working around the kind of injury that Burkhart suffered to actually restoring the broken connections in his body.
Rebuilding brains
The idea is more than just an academic dream. US start-up Science Corporation is dedicating significant resources to building a commercial biohybrid implant that it hopes could someday help repair connections in the most complex organ of all: the brain.
Alongside conventional brain implants and a retinal implant designed to restore sight, the company is developing a new brain implant that it hopes will surpass any silicon chip in terms of the data it can handle.
The device starts with a honeycomb-like scaffold that nestles on the brain’s surface. In each of its more than 100,000 tiny compartments sits a single neuron that has been genetically engineered to respond to light. Each gap in the honeycomb contains a tiny light source to activate the neuron and an electrode to record signals returned from the brain. Once implanted, the neurons would extend filaments that connect to neurons in the organ.
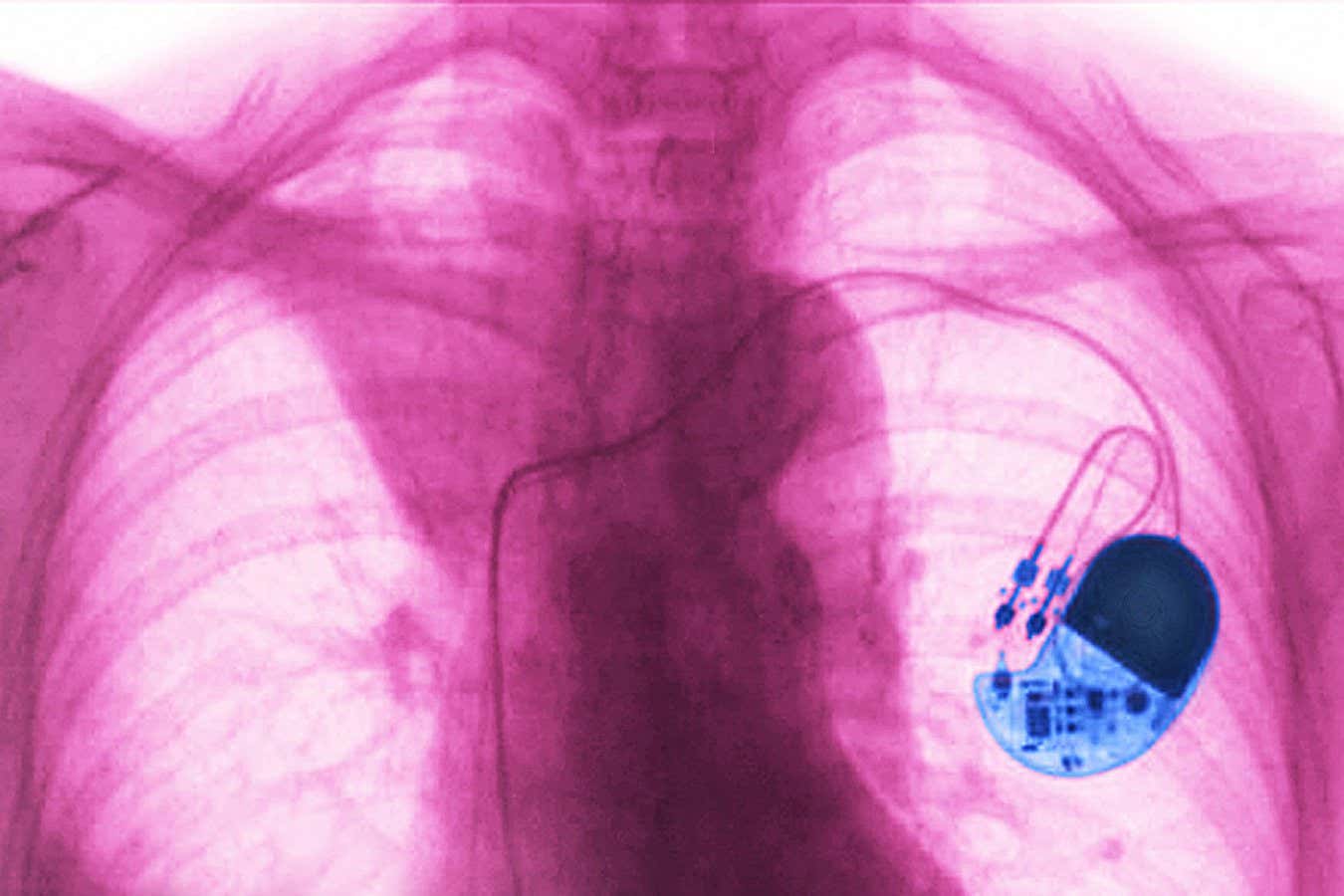
Pacemakers treat abnormal heart rhythms by sending small electrical pulses to cardiac muscles.
Cavallini James/BSIP/Universal Images Group
So far, this device has demonstrated the ability to send basic signals to a rodent’s brain in animal trials. But the long-term hope is that, one day, these individually controllable neurons could create general-purpose implants with many more connections than are currently feasible. Alan Mardinly, Science Corporation’s director of biology, says he doubts that conventional implant technologies will be able to get past a few tens of thousands of connections, but future biohybrid devices could reach the millions.
That could be transformative. Think of a patient who has a stroke and loses the ability to speak, says Mardinly. This may damage a brain region, but an implanted lattice of neurons, trained with patterned light, could take over its function. “We view it basically as putting brain-computer interfaces on a spectrum with regenerative medicine,” he says.
Living electronics
Burkhart, who received his implant over a decade ago, has seen the promises and limitations of these experimental brain devices up close. He isn’t won over by all the developments that people like Mardinly say are still a long way off. “I originally had my [device] implanted over 11 years ago,” he says. “If you had asked me then where we’d be now, I would have thought we’d have a lot more success.”
But Burkhart isn’t sitting on his hands. He has assembled a group of early brain-computer interface (BCI) research participants called BCI Pioneers to ensure that big innovations in the field remain practical and scalable for real users. And he thinks brain implant technology has reached a tipping point, moving out of labs and into people like him.
Meanwhile, at the University of Pennsylvania, Kacy Cullen is working on what may be the boldest concept yet: throwing out the electronics entirely. He is developing what he calls “living electrodes” – devices built wholly from cells and tissue scaffolds, designed to integrate with the brain and function like native neural circuits.
“
These engineered neuronal microtissues could potentially expand the computational capacity of the brain itself
“
Each consists of a clump of neurons genetically engineered to respond to light, perched on a slender hydrogel cylinder. The structure is implanted into the brain like a hollow rod, with the neural bundle remaining at the brain’s surface. Over time, these neurons extend long filaments called axons down the tube, connecting with the cells deep in the host’s brain. It is a biological relay that could bridge different brain regions or link the brain to external devices, like prosthetics or computers, all without triggering the immune response that dooms traditional implants.
And unlike electrodes made of metal, which can record inputs from only a handful of neurons, each biological cell can form thousands of connections. Different neuron subtypes use distinct neurotransmitters – chemicals that transmit signals – and form different types of junctions between brain cells, known as excitatory or inhibitory synapses, a variety that offers a richness and specificity that silicon can’t rival. This could allow for a much finer level of control and potentially a higher bandwidth for communication to and from the brain via such a device, says Cullen.
But why stop there? “These engineered neuronal microtissues could potentially expand the computational capacity of the brain itself, effectively adding new layers of information processing to the nervous system,” he says. In theory, that means more than just repairing damaged circuits – it could one day enhance memory or learning.
For now, though, this remains a distant goal, and the applications are more grounded. Cullen’s lab received a grant from the UK’s Advanced Research and Invention Agency (ARIA) to adapt the technology for Parkinson’s disease. The aim is to rebuild a key neural circuit – the nigrostriatal pathway – that degenerates from the condition, depriving the brain of dopamine, a neurotransmitter.
His collaborators, led by Flavia Vitale at the University of Pennsylvania, are creating a biosensor that measures dopamine production in real time. This signal would feed into the living electrode, prompting it to adjust the amount of dopamine it produces.
The approach could have applications beyond Parkinson’s, says lab member Dimitris Boufidis, because many neurological and psychiatric disorders feature similar disruptions to brain circuitry. “You can think of it as bridges connecting different parts of the brain that get destroyed,” he says. “What if we could rebuild these bridges?”
Green’s team at Imperial has also received funding under the same ARIA grant to create a scaffold, seeded with neuronal stem cells, that can be injected into the brain to repair damaged circuits. Light and electricity will then be used to guide neuron growth and connections. Though an underlying disease could eventually also degrade repaired connections, Green says it is worth it. “At the end of the day, you buy people time and you buy people quality of life,” she says.
Her lab is also exploring whether the same approach could help with traumatic brain injuries and spinal cord damage, like Burkhart’s. “[We’d use] electrical stimulation to drive regeneration and drive connection, rather than bypassing the injury,” she says.
Though Burkhart remains realistic about the time these things can take to reach patients, it is an approach that resonates with him. “If we can get something that allows someone to function the way they were able to function before a disease or injury, and just allowing them to be as independent as possible, that would be a huge benefit for those individuals and society as a whole,” he says.
Topics:
Source link : https://www.newscientist.com/article/2492875-the-futuristic-new-tech-that-could-bridge-broken-nerves-and-mend-minds/?utm_campaign=RSS%7CNSNS&utm_source=NSNS&utm_medium=RSS&utm_content=home
Author :
Publish date : 2025-09-03 16:00:00
Copyright for syndicated content belongs to the linked Source.