ORLANDO — First-dose reactions to IV ustekinumab (Stelara) may be related to IgE recognition of a carbohydrate allergen in patients, who may be able to tolerate subcutaneous ustekinumab instead, according to a small study.
The biologic seemed to express an epitope to alpha-1,3-galactose (alpha-gal) that IgE antibodies recognize in people with alpha-gal syndrome (AGS), reported Preethi Venkat, MD, MPH, of the University of Virginia School of Medicine in Charlottesville, at the Advances in Inflammatory Bowel Diseases annual meeting. AGS makes people allergic to red meat and other mammalian products.
In a poster presentation, the authors described six patients with Crohn’s disease from two academic clinics who had a first-dose reaction to ustekinumab and had detectable IgE specific to alpha-gal in their serum. Two patients were biologic-naive and had never had a reaction to a medication. Of three who had previously taken adalimumab (Humira), one had experienced papular dermatitis in response to adalimumab, and another, who had also previously received infliximab (Remicade), had experienced serum sickness in response to adalimumab.
The sixth patient with no prior history of medication reactions had previously taken infliximab, adalimumab, vedolizumab (Entyvio), and ustekinumab but had stopped taking ustekinumab 18 months earlier because of a change in insurance.
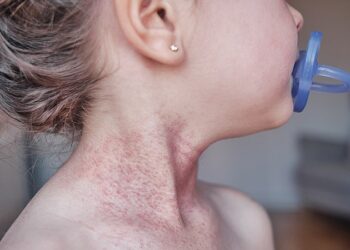
All of the patients developed urticaria when first administered IV ustekinumab but were then later able to tolerate subcutaneous ustekinumab. Their levels of IgE specific to alpha-gal ranged from 1.88 to 13.6 IU/ml, Venkat and colleagues reported.
“For patients with mild to moderate hypersensitivity reactions to IV ustekinumab, switching to subcutaneous and considering referral to an allergist for monitored administration is advisable,” they wrote. “Further studies on IgE binding and basophil activation across monoclonal antibody [mAb] concentrations could clarify dose-response relationships and guide safe use of these mAbs in alpha-gal syndrome patients.”
An estimated 0.9-4.5% of patients receiving IV ustekinumab experience a hypersensitivity reaction to the first dose, but it hasn’t been clear what’s causing the reaction, the authors noted. They also pointed out that previous research has suggested that IgE recognition of alpha-gal is linked to both mammalian meat allergy and to first-dose anaphylactic reactions to cetuximab.
“Ustekinumab, like cetuximab, is produced in murine Sp2/0 cell lines, which have been shown to preferentially add alpha-gal glycans,” the authors stated. “This glycosylation of alpha-gal is not seen in Chinese hamster ovary cell lines, which are commonly used in the development of other monoclonal antibodies.”
They sought to test whether pre-existing IgE to alpha-gal might explain why some patients experience a first-dose reaction to ustekinumab.
They used an ImmunoCAP-based assay to test the serum of an unrelated cohort of four patients with alpha-gal syndrome to see if their serum had IgE that could bind to ustekinumab, cetuximab, and infliximab.
In serum samples with no beef thyroglobulin present, all of the patients’ IgE bound to cetuximab, ustekinumab, and infliximab. As the concentration of beef thyroglobulin increased, from 2.5 to 2,500 μg/ml, the proportion of IgE specific to each biologic decreased, but the decrease was more dramatic in ustekinumab and infliximab while cetuximab-specific IgE levels were comparatively higher. IgE specific to ustekinumab and infliximab fell to virtually undetectable levels in serum with the highest concentrations of beef thyroglobulin.
“Serum-based ImmunoCAP testing in patients with alpha-gal syndrome, but not controls, revealed high levels of IgE binding to cetuximab and moderate binding to ustekinumab and infliximab, which is also produced in Sp2/0 cell lines,” the authors reported. When the researchers tested these patients’ sera against vedolizumab, a biologic that is produced using Chinese hamster ovary cell lines, no reactivity (no IgE binding) occurred.
Malcolm Irani, MD, of Houston Methodist Gastroenterology Associates, said that he found the results particularly interesting because he had only been familiar with polysorbate 80 allergy to biologics. He said these findings potentially helped explain a clinical situation he had seen.
First-dose reactions to ustekinumab are relatively rare, he told MedPage Today, but “they can certainly limit our use.” He said that when someone has a reaction, it can often lead a clinician to take a step back to consider whether the patient may have an allergy to polysorbate 80, a surfactant used in a majority of biologics. A polysorbate 80 allergy would substantially limit a provider’s use of nearly all biologics available, suggested Irani, who was not involved in the study.
“When we initially see people that have a reaction, our first thought is polysorbate 80,” which can then cause hesitancy in prescribing any other biologics, he said. “But the poster is saying [the cause of the reaction] could be something else.”
“I have a patient that had a first-dose reaction to ustekinumab, but he was able to tolerate vedolizumab before,” he said. “I told him, ‘It’s probably not polysorbate 80, but I don’t know what caused this reaction.’ So maybe it’s this galactose alpha 1-3.”
He said these findings are encouraging in that they suggest a patient who initially has a reaction to IV ustekinumab may still be eligible to try other biologics if their reaction is not due to polysorbate 80.
“I think if I ever come across somebody else that has an allergy to ustekinumab, I’ll pause and take a step back before going down that polysorbate 80 [route] because I think that’s our first stop,” he said. “Now I have something else to think about, which will open my window to other therapeutic options on the line.”
Disclosures
Irani disclosed no relationships with industry.
Primary Source
Advances in Inflammatory Bowel Disease
Source Reference: Venkat P, et al “First-dose infusion reaction to ustekinumab is associated with the presence of serum IgE against galactose-a-1,3-galactose (a-gal)” AIBD 2024; Abstract S39.
Source link : https://www.medpagetoday.com/meetingcoverage/aibd/113394
Author :
Publish date : 2024-12-14 21:47:18
Copyright for syndicated content belongs to the linked Source.


![author['full_name']](https://newshealth.biz/wp-content/uploads/2024/12/Alpha-Gal-May-Drive-Sensitivity-to-IV-Stelara-in-People-With.jpg)