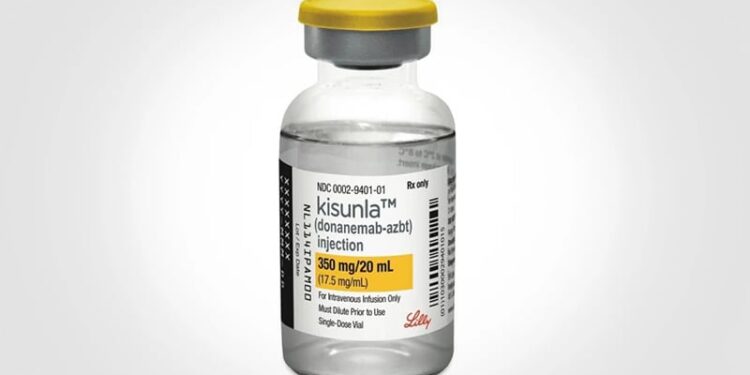
The European Medicines Agency (EMA) has recommended refusal of a marketing authorization for donanemab, which targets the treatment of early Alzheimer’s disease (AD). The agency’s Committee for Medicinal Products for Human Use said that the benefits of the medicine were not sufficient to outweigh the risk of potentially fatal events.
Donanemab is a monoclonal antibody against amyloid beta, intended to slow the progression of AD in adults with mild cognitive impairment or mild dementia by reducing amyloid plaques in the brain. It is given as a monthly infusion in early symptomatic disease, but frequently causes amyloid-related imaging abnormalities (ARIA), which represent swelling and potential bleedings in the brain.
Fatality Risk Outweighs Benefits
While this does not usually cause any symptoms and is usually a temporary effect, more extensive bleeding and serious symptoms can occur. These can include headache, dizziness, nausea, difficulty walking, confusion, vision changes, and seizures. In severe cases, ARIA can be fatal, and the committee considered that this risk outweighed the potential benefits of the drug.
Marketing approval had been sought based on data from the TRAILBLAZER-ALZ 2 clinical trial, which compared donanemab with placebo in 1736 patients with early symptomatic AD (prodromal AD and mild dementia due to AD) and demonstrated brain amyloid beta plaques and tau pathology. At the main 76-week end point, integrated AD rating scale scores, which measure both cognitive and functional ability, had worsened by only 10 points in patients who received donanemab vs 13 points in those on placebo. The trial included patients both with and without copies of the apolipoprotein E4 (ApoE4) gene variant. In participants without ApoE4, these figures were 14 vs 16, respectively.
Risk Affected by Gene Profile
Donanemab was approved by the US Food and Drug Administration in July last year. The mode of action of donanemab is similar to that of lecanemab, which was approved by the EMA last November for a restricted group of patients with only one or no copies of ApoE4, overturning a previous negative opinion in a broader patient population. People with one or two copies of this gene are known to be at higher risk of developing serious side effects with this group of medicines. During the current application for donanemab, the company had proposed restricting its use to patients who do not have ApoE4.
However, the EMA has now said that the benefits of donanemab were not large enough to outweigh the risks of potentially fatal events due to ARIA, even in the small group of people who do not carry copies of ApoE4. The committee noted that ARIA emerged as the most important safety concern in the TRAILBLAZER trial, occurring in 36.8% of the active group compared with 14.9% of the placebo arm. Although most cases did not involve symptoms, serious ARIA events occurred in 1.6% of the donanemab-treated patients, resulting in death in three cases. Even in the smaller group of participants with no copies of ApoE4, ARIA occurred in 24.7% of the donanemab group compared with 12% of the placebo group, and serious ARIA events still occurred in 0.8% of the active group, leading to one death.
Unmet Need Remains
In reaching its conclusion, the EMA acknowledged the unmet medical need for AD treatments, and took into consideration the views of patients, healthcare professionals, a scientific advisory group including neurologists, and healthcare professional organizations.
The manufacturer has 15 days to ask for a re-examination of the opinion.
Source link : https://www.medscape.com/viewarticle/alzheimer-drug-donanemab-gets-no-go-european-regulator-2025a10007j6?src=rss
Author :
Publish date : 2025-03-28 15:36:00
Copyright for syndicated content belongs to the linked Source.