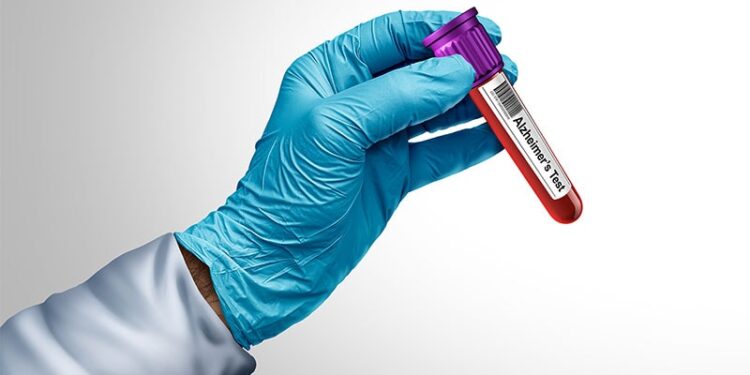
OTTAWA, Ontario — Looking for Alzheimer’s disease (AD) biomarkers in plasma instead of cerebrospinal fluid (CSF) is a simpler form of screening individuals for AD pathology and could result in earlier delivery of anti-amyloid therapies to symptomatic individuals, according to Mari DeMarco, PhD, clinical chemist at St. Paul’s Hospital and clinical professor of pathology and laboratory medicine at the University of British Columbia, both in Vancouver.
Obtaining blood to detect biomarkers of AD is less invasive than obtaining CSF, DeMarco said during a presentation at the Canadian Neurological Sciences Federation Congress 2025. “We know from decades of research that this pathology leaves a signature behind in CSF,” she said. “We now know that it also leaves a signature behind in blood.”
One AD biomarker that is more easily identified than others in plasma is phosphorylated tau 217, noted DeMarco.
Biomarker identification has undergone many advances, such as the development of mass spectrometry. “With CSF, those early assays had high variability and questionable accuracy,” said DeMarco. “There was a big push in the field to develop accurate assays, which led to mass spectrometry. That is the gold standard.”
One of the difficulties of analyzing biomarkers in plasma is the need for careful collection and care of samples. “Getting them [plasma samples] to the analyzer in a safe and proper way so that we can accurately quantify [biomarkers] can be a challenge,” said DeMarco. The long-term stability of the plasma biomarkers in storage, which affects their diagnostic accuracy, is another potential concern, she added.
Identifying biomarkers in CSF requires lumbar punctures, and using a blood test would increase the accessibility of screening for AD pathology. De Marco nevertheless called for caution about making plasma biomarker testing widely available in primary care.
“If you are getting testing to confirm that you have the pathology associated with AD for the purpose of getting an anti-amyloid therapy, then the person ordering the test should probably be the one who is going to help manage the patient,” DeMarco told Medscape Medical News. A hybrid model of AD care that involves primary care physicians and specialists may be the solution to incorporating blood biomarkers in clinical practice, she added.
Plasma biomarkers may have a negative predictive value to rule out AD, as well as a positive predictive value, said De Marco. Depending on the test result, confirmatory testing may be needed in the form of another blood test, CSF test, or PET scan.
Pardh Chivukula, MD, a neurologist at St. Michael’s Hospital in Toronto and vice president of the Canadian Neurological Society in Calgary, suggested that it might be beneficial to healthcare delivery in Canada if, in a few years, patients could be triaged in primary care through testing for AD biomarkers in plasma. The appropriate patients could be referred to specialist care.
“The field is still very early on in development,” said Chivukula told Medscape Medical News. “That’s certainly a good goal for us to have in the future. And I think potentially it can streamline the efficiency of our healthcare system if we can use these biomarkers to screen for which patients are a higher risk, or more likely to have these conditions, using simple blood tests.”
DeMarco reported serving on advisory boards for Roche, Eisai, and Siemens, and her institution received in-kind support from Roche, Fujirebio, and Meso Scale Diagnostics. Chivukula reported having no relevant financial relationships.
Source link : https://www.medscape.com/viewarticle/alzheimers-disease-leaves-signature-blood-and-csf-2025a1000g4f?src=rss
Author :
Publish date : 2025-06-17 07:34:00
Copyright for syndicated content belongs to the linked Source.