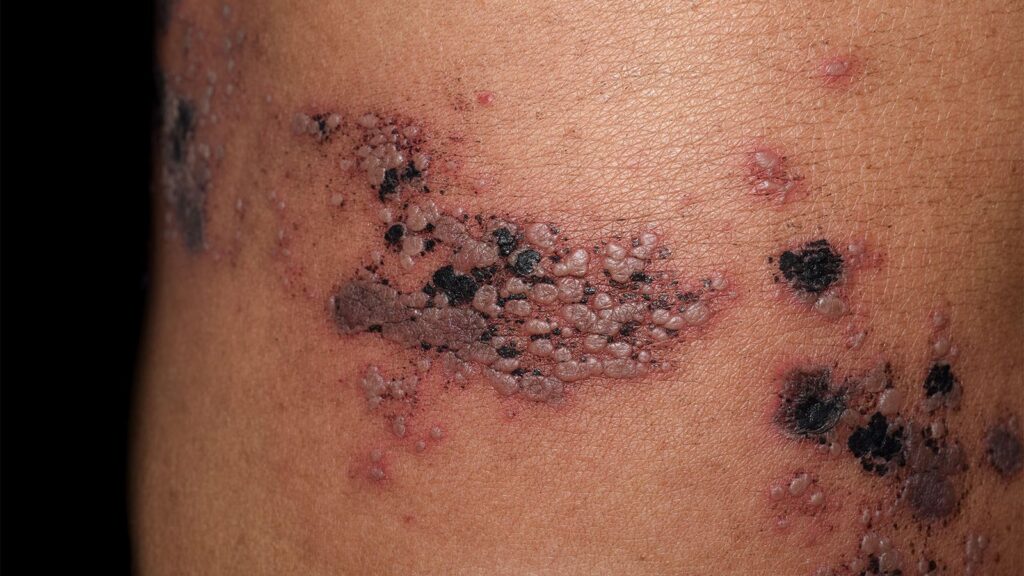
The antiviral drug valaciclovir (Valtrex), given prophylactically, kept patients with systemic lupus erythematosus (SLE) under treatment with anifrolumab (Saphnelo) from having herpes zoster attacks, researchers said, offering a potential alternative to zoster vaccination.
Among 87 anifrolumab users given valaciclovir, none developed herpes zoster attacks (also known as shingles) after median follow-up of 234 days, whereas four cases developed among 45 patients not co-treated with the antiviral agent, according to Ludovic Trefond, MD, PhD, of Université Clermont Auvergne in Clermont-Ferrand, France, and colleagues.
That translated to a hazard ratio of 0.08 (95% CI 0.01-0.59) in univariate analysis, the group reported in RMD Open. Notably, three of 13 patients (23%) not receiving valaciclovir who had at least 12 months of follow-up suffered attacks.
Trefond and colleagues wrote that the study “suggests that prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab. This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable.”
Untreated SLE comes with increased risk for zoster attacks, and it’s even higher with anifrolumab. Clinical trial data showed attack rates of 6.1% with the drug, which blocks interferon from activating its receptors, versus 1.3% in the placebo groups. Most attacks occurred during the first year of anifrolumab, Trefond’s group observed.
In general, vaccinations against various infectious diseases are recommended for lupus patients. But for a long time, the only adult vaccine for herpes zoster was a live-attenuated virus product (Zostavax), which is contraindicated with anifrolumab because the latter’s anti-interferon effect may allow the attenuated virus to replicate rapidly and cause disseminated infection.
A recombinant vaccine that uses only certain herpes zoster proteins is now available (Shingrix) and is considered safe to use with anifrolumab. Still, zoster vaccine uptake in SLE patients remains dismally low: 4.9% (two patients out of 41) in a study reported in 2023.
The French study used observational data provided by numerous centers in the country. Patients on anifrolumab for at least 3 months between November 2021 and July 2024 were included. Prophylactic valaciclovir was prescribed at clinicians’ and patients’ discretion; 14.9% of those given the drug had a previous history of shingles, versus none of those not receiving it. The 87 given valaciclovir included two who had received the live-attenuated zoster vaccine; none had had the recombinant product.
Some three-quarters of valaciclovir prescriptions were for 500 mg/day; the rest got 1,000 mg/day. It was intended to be taken as long as anifrolumab was being administered. Fourteen patients stopped anifrolumab while under observation, including 10 for inefficacy, two to try getting pregnant, and two because of infection.
Overall, the 132 patients reflected the general profile for SLE: mean age was 42 and just over 90% were women. Median disease duration was about 13 years in the valaciclovir group and 15 years for the no-treatment group. In addition to anifrolumab, most patients were also using standard lupus medications including hydroxychloroquine, prednisone, methotrexate, mycophenolate mofetil, and/or azathioprine. A handful were on belimumab (Benlysta) as well as anifrolumab. Those on versus not on valaciclovir were generally similar, except for history of previous zoster attacks.
Limitations to the analysis included the retrospective and non-randomized design and the small number of herpes zoster attacks seen during follow-up.
Disclosures
The study had no outside funding.
Trefond reported relationships with GSK, AstraZeneca, Otsuka, and CSL VIFO.
Co-authors reported relationships with numerous pharmaceutical and vaccine companies and other commercial entities.
Primary Source
RMD Open
Source Reference: Trefond L, et al “Efficacy of valaciclovir in preventing herpes zoster in patients receiving anifrolumab” RMD Open 2025; DOI: 10.1136/rmdopen-2024-005076.
Please enable JavaScript to view the comments powered by Disqus.
Source link : https://www.medpagetoday.com/rheumatology/lupus/113664
Author :
Publish date : 2025-01-06 21:45:53
Copyright for syndicated content belongs to the linked Source.