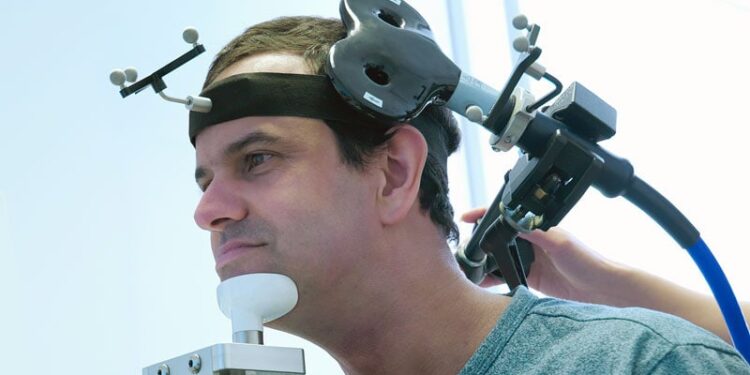
Transcranial magnetic stimulation (TMS) may augment standard language therapy to help slow the progression of primary progressive aphasia (PPA), a neurodegenerative disorder that erodes communication.
In a randomized sham-controlled clinical trial, 6 months of active intermittent theta-burst TMS
plus language therapy improved or mitigated decline in regional brain metabolism, trained language abilities, functional impairment, and neuropsychiatric symptoms in adults with PPA.
The study was published online on August 11 in JAMA Network Open.
Added Value?
PPA is a heterogeneous clinical syndrome marked by progressive speech and/or language impairment. Most cases stem from frontotemporal degeneration or Alzheimer’s disease. There are currently no effective drug treatments, although speech-language therapy has proven to be helpful.
TMS can induce changes in cortical excitability, potentially promoting the reorganization of language networks, and has shown promise as adjunctive treatment for post-stroke aphasia.
Previous studies examining the short-term effects of TMS on PPA reported “encouraging” results, but the longer-term effects, beyond more than a few weeks of intervention, have not been examined, until now.
For the study, the researchers led by Jordi Matias-Guiu, MD, PhD, with the Department of Neurology, Hospital Clínico San Carlos, Madrid, Spain, recruited 63 adults with early-stage PPA (42 women; mean age, 72 years).
Participants were randomized (2:1) to either active intermittent theta-burst TMS or sham TMS for 6 months, immediately followed by evidence-based language therapy for PPA.
The trial consisted of a 24-week treatment that included a 2-week intensive course, in which active TMS or sham TMS combined with language therapy was applied daily, followed by a maintenance phase in which the same stimulation was applied weekly for 22 weeks. Participants were assessed at baseline, 3 months after the start of the treatment, and at the end of the treatment (6 months following baseline).
The main outcome was the standardized uptake value ratio (SUVR) on FDG PET imaging in the left hemisphere, assessed at baseline and at 6 months (immediately following the intervention).
Feasible, Effective Option
The primary outcome was positive with higher adjusted mean SUVR in the active group than in the sham TMS group (0.78 vs 0.77; P = .046).
Active TMS was also associated with significant improvement on all secondary language outcomes at 6 months, including the mini linguistic state examination, with an adjusted mean difference at 6 months of 7.71 (P = .002). Patients in the sham group worsened on this measure compared with those in the active group.
Active TMS also led to improvement in confrontation naming of trained words, which improved by a mean 23.8 points in active recipients compared with sham TMS recipients.
Functional independence also benefited, with daily-living scores falling (indicating better performance) by 5.4 points in the active group compared with the sham TMS group. Neuropsychiatric symptoms eased as well, with a 4-point advantage on the neuropsychiatric inventory scale.
There were no significant differences in the number of adverse events. Adherence to treatment protocol was high (92%).
“Overall, these findings suggest that the combination of TMS and language therapy is a feasible and effective treatment option for PPA,” the researchers concluded.
They said future studies should investigate the potential for TMS paired with an evidence-based speech-language intervention to sustain or extend these benefits beyond 6 months.
This study had no commercial funding. The authors declared no relevant conflicts of interest.
Source link : https://www.medscape.com/viewarticle/brain-stimulation-promising-primary-progressive-aphasia-2025a1000lah?src=rss
Author :
Publish date : 2025-08-12 06:33:00
Copyright for syndicated content belongs to the linked Source.