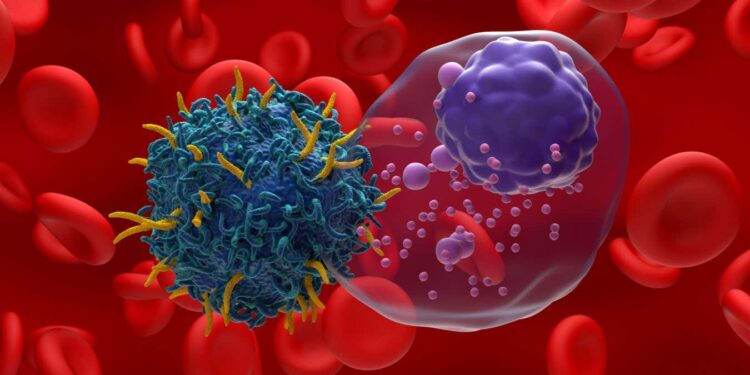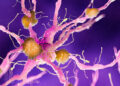
Illustration of CAR T-cell therapy targeting multiple myeloma, a type of blood cancer
Nemes Laszlo/Alamy
CAR T-cell therapy has the potential to revolutionise how we treat certain types of cancer by genetically engineering someone’s own immune cells to attack the disease – but it is also cumbersome and expensive. Now, scientists have managed to create the bespoke therapy inside the body of non-human animals, which could simplify the process, driving costs down.
The treatment is mainly available in countries including the UK and US for some people with various types of blood cancer, including some types of leukaemia, where B-cells, a part of the immune system, grow uncontrollably. In these cases it involves collecting a sample of immune cells called T-cells from the blood of a patient and genetically engineering the genome within those cells to permanently target and kill B-cells. These engineered T-cells are then multiplied and infused back into the body.
But this process takes time. “You’ve got to take blood, ship it to a central manufacturing lab and then return [the T-cells],” says Carl June at the University of Pennsylvania. “That means it’s very hard to scale up.” It can also cost upwards of $500,000 per patient.
To develop a more efficient approach, June and his colleagues turned to genetic molecules, in this case RNA, that carried instructions to make a protein that recognises B-cells. They packaged these molecules into fatty capsules, which were coated in a protein that allows them to get into T-cells, which then gain the ability to recognise and destroy B-cells. This effect is only temporary, however, as the RNA code remains in the T-cells for about a week before degrading.
Putting their approach to the test, the researchers infused cancerous human B-cells and healthy human T-cells into mice that had been bred to lack an immune system. One week later, they injected the animals with five doses of the fatty capsules across roughly two weeks, with some mice receiving higher doses than others.
Three weeks later, the mice that received the highest dose had no detectable tumour cells and experienced no side effects. “Tumour cell levels were as close as we can detect to being eliminated,” says June.
The team also injected the fatty capsules into 22 healthy monkeys. This generated CAR T-cells in the body that completely cleared all B-cells within just one day, which suggests that the approach could also treat this type of leukaemia in primates. B-cells, which make antibodies, are an important part of the immune system, yet the treatment was well tolerated in all but one of the monkeys, which had a severe inflammatory reaction.
“This is really impressive,” says Karin Straathof at University College London. It is a potentially much simpler procedure for making CAR T-cell therapy, which could make it more affordable, she says.
But one benefit of traditional CAR T-cells is that they can offer long-term protection, says Straathof. The new approach only temporarily produces such cells, so further injections would be needed if the cancer returns. We also can’t be sure of the latest method’s effectiveness or safety in people without clinical trials, she says.
June says the team is already testing the approach in healthy humans. “The first person was dosed in the past few weeks,” he says.
Topics:
Source link : https://www.newscientist.com/article/2485142-car-t-cell-therapy-could-be-made-in-the-body-of-someone-with-cancer/?utm_campaign=RSS%7CNSNS&utm_source=NSNS&utm_medium=RSS&utm_content=home
Author :
Publish date : 2025-06-19 19:00:00
Copyright for syndicated content belongs to the linked Source.