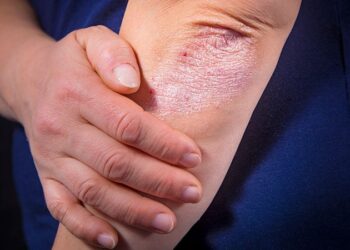
A derivative of the diuretic drug amiloride (Midamor) blocked key inflammatory pathways in mouse models of psoriasis, including xenografts of psoriatic human skin, as well as in cultured patient skin samples, researchers said.
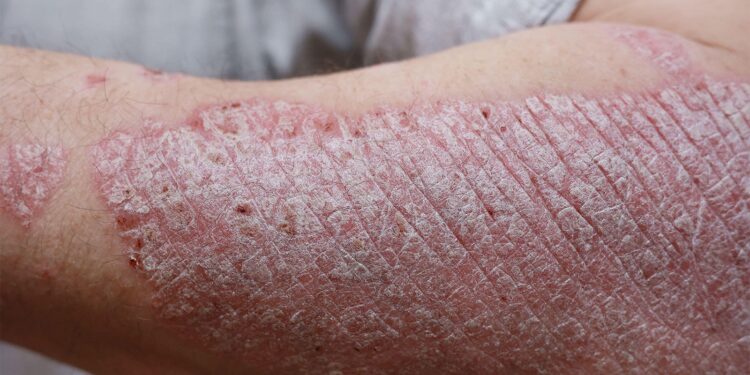
Skin cells taken from human psoriasis patients, reconstructed into tissue and grafted onto immunodeficient mice, “showed pronounced psoriasiform hyperplasia” when treated with a placebo solution, but when benzyl amiloride was applied, no such abnormal growth was seen, according to M. Peter Marinkovich, MD, of Stanford University in Palo Alto, California, and colleagues.
In addition, experiments with cultured psoriatic skin cells showed that the agent prevented cell growth driven by epidermal growth factor, the group reported in Science Translational Medicine. The live-animal studies also showed that benzyl amiloride, also known as benzamil, effectively inhibited skin inflammation when given systemically as well as topically.
These studies “demonstrate the potential of the sodium channel inhibitor benzamil as a prototype for an effective, cost-efficient, and targeted therapeutic for skin inflammation,” Marinkovich and colleagues wrote. “Future studies will ultimately determine the tolerability and efficacy of sodium channel inhibitors for translating these findings to the clinic.”
The investigators didn’t begin by thinking that a sodium channel blocker would be effective in psoriasis. Rather, they ended up with it through a computational analysis of previously published gene expression profiles for existing psoriasis treatments, the results of which (called Prototype Ranked Lists or PRLs) were then compared with expression profiles generated from psoriasis patients’ cells, also compiled into PRLs. It’s then possible to identify existing drugs that might work to oppose pathologic gene expression.
Thus, the group explained, “a meta-analysis of publicly available datasets from disease PRLs of lesional skin of 200 patients with psoriasis was compared with that of drug PRL gene expression signatures. This identified the drug benzamil … as a therapeutic candidate. Benzamil showed a marked anticorrelation with psoriasis, which implicated down-regulation of pathways involving epidermal signaling and epidermal-immune cross-talk.”
Benzamil is not itself an FDA-approved drug, but it’s widely available for research purposes. It’s considerably more potent than amiloride, which, in the above analysis and also in the published literature, had no suggestion of being effective in psoriasis. As the authors noted, benzamil’s potential toxicity as well as clinical efficacy obviously needs to be explored in additional studies.
Key to the drug’s effect is its inhibition of a pathway involving Rac1, shorthand for Ras-related C3 botulinum toxin substrate 1. “Previous work has shown that epidermal-specific Rac1 and STAT3 activation can promote abnormal IL [interleukin]-17- and IL-23-mediated immune activity and induce a phenotype in mice closely resembling human psoriasis,” the researchers wrote. In 2016, a group with many of the same authors reported another xenograft study showing that inhibiting Rac1 in a different way was able to “rescue” psoriatic cell growth and inflammation.
Overall, this line of research and the new benzamil study indicate that “modulation of sodium flux in keratinocytes can regulate Rac1 activity by decreasing submembranous pH.”
Marinkovich and colleagues remarked that further tweaks to the benzamil molecule might lead to an even better drug candidate. Even as is, benzamil is particularly attractive as a topical treatment, they wrote, since it should be safer than the “high-potency steroids” now often used to control psoriatic lesions and would also lack unwanted systemic immunosuppression.
Disclosures
The study was funded by the National Institute of General Medical Sciences.
Several co-authors reported serving as co-founders and/or holding stock in companies licensing technology related to the study; four of them are listed as inventors on patent applications for benzyl amiloride as a psoriasis therapy. Others reported relationships with numerous established pharmaceutical and electronics companies.
Primary Source
Science Translational Medicine
Source Reference: Winge MCG, et al “Repurposing an epithelial sodium channel inhibitor as a therapy for murine and human skin inflammation” Sci Transl Med 2024; DOI: 10.1126/scitranslmed.ade5915.
Source link : https://www.medpagetoday.com/dermatology/psoriasis/113348
Author :
Publish date : 2024-12-11 21:36:13
Copyright for syndicated content belongs to the linked Source.

![author['full_name']](https://newshealth.biz/wp-content/uploads/2024/12/Could-Psoriasis-Treatment-Be-This-Simple.jpg)