The medical community is accustomed to rigorous standards for drug development and approval. But in the context of assisted dying, there is a surprising and persisting lack of robust scientific data.
“It’s very hard to do scientific research with regard to the usage of drugs for euthanasia. If you apply euthanasia, you want to be successful, and you can’t use any other drugs than the drugs we know work,” Steven Pleiter, former managing director of the Dutch Center of Expertise for Euthanasia, told Medscape Medical News. “But the evidence is based on years and years of experience.”
“The amount of evidence supporting the use of these drugs is astoundingly small,” Claud Regnard, MD, a retired palliative medicine consultant in the United Kingdom, told Medscape Medical News. “The last study looking at efficacy and side effects was published 25 years ago, using data from 10 years earlier.”
The Drugs and Their Protocols
The Netherlands, one of the first European countries to legalize euthanasia and assisted dying, has developed guidelines on their implementation, now in their third edition.
Euthanasia is when a doctor directly ends the life of a patient, while assisted dying is when a doctor provides the means for them to end their own life.
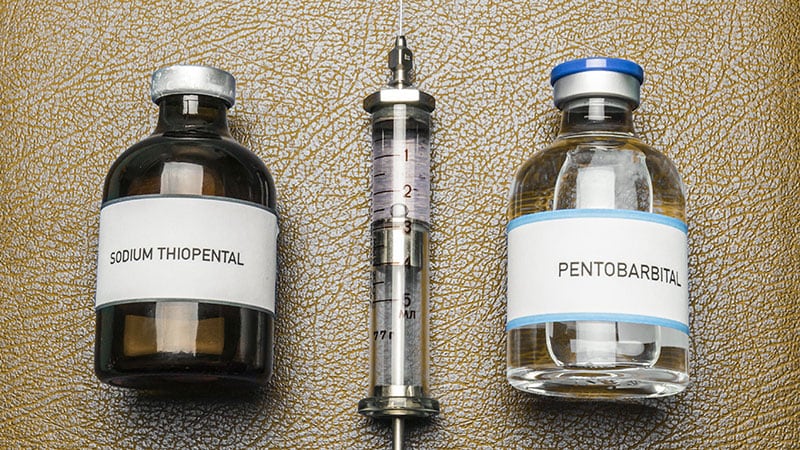
For euthanasia, the standard Dutch protocol involves an initial injection of thiopental or propofol at doses several times higher than those used in general anesthesia to induce a deep coma. This is followed by administering a neuromuscular blocking agent such as rocuronium, atracurium, or cisatracurium in doses sufficient to cause complete paralysis and eventual death. “Most people die after the coma-inducing drug because it’s such a high dose,” Pleiter said. “The patient will die within seconds. It’s very rapid.”
Assisted suicide in the Netherlands involves the oral ingestion of pentobarbital or secobarbital in doses far exceeding what would be used for sedation, often flavored to mask the taste. An antiemetic is administered 24 hours before the ingestion of the barbiturate. “The oral pathway can take a few hours. The doctor remains with the patients and will administer an intravenous dose if the patient has not died after 1 hour,” he explained. The vast majority of cases (97%) undergo euthanasia and thus use intravenous drugs. Only 3% undergo assisted dying involving oral ingestion, Pleiter said.
In Switzerland, a commonly used drug is the fast-acting barbiturate sodium pentobarbital, according to documentation provided by Dignitas to Medscape Medical News. This is usually taken orally or, in some cases, via a gastric tube or intravenously. The documentation did not include specific data on this drug’s efficacy or complication rates. Dignitas declined a request for an interview.
In Italy, where assisted dying is a more recent development and only a handful of cases have occurred, Mario Riccio, MD, a retired anesthetist, current advisor of the Luca Coscioni Association, and the doctor involved in most Italian cases, reported using intravenous drugs and dosages based on the Dutch guidelines and personal experience. “I know these drugs are used elsewhere. I know they are safe. I know they work,” he told Medscape Medical News. He maintains that, while minor complications are possible, they are less significant for individuals facing terminal suffering and seeking a dignified end.
Unlike the Dutch protocol, however, he cannot subsequently administer neuromuscular blockers because patients must self-administer the drugs under Italian regulations, which he views as unnecessarily burdensome. Once the barbiturates take effect, patients are no longer able to administer a second drug. “A neuromuscular blocker would provide an extra layer of efficacy, but I am not allowed to touch the patient during the procedure,” he explained.
There is no standardized global approach to drug selection and dosing for either euthanasia or assisted dying, and the process is mainly empirical. “There isn’t a single drug regulatory authority anywhere in the world that has assessed and approved assisted dying drugs [in the doses required for this purpose],” said Regnard.
Instead, these medications are approved for indications such as anesthesia or epilepsy, and their use in euthanasia or assisted dying falls under off-label prescribing. Physicians rely on guidelines established by medical associations, expert committees, and historical clinical practice for their use.
The Evidence Gap
Unlike other areas of medicine, assisted dying has largely escaped rigorous scientific evaluation. “You wouldn’t allow this in any way with any other sort of drugs,” Regnard said. In a 2022 study, he found that drugs used for assisted dying have not undergone the usual level of scrutiny.
The pharmacokinetics and pharmacodynamics of these drugs at high doses remain poorly understood. “We extrapolate from therapeutic doses, but we have no proper data on what happens at lethal doses,” Regnard said. “That’s not science — that’s guesswork.”
He said most jurisdictions, like Switzerland, the Netherlands, Belgium, Canada, and Australia, do not systematically collect or publish data on assisted dying drug efficacy, mechanisms, and complications. “Oregon is the only jurisdiction providing some transparency, but even their data is severely incomplete,” he said.
In a 2023 report from the US state of Oregon, 74% of complication data were missing. Of the available data, 9%-11% of patients experienced complications, including vomiting, aspiration, agitation, and seizures. “In some cases, patients regained consciousness after ingesting a lethal dose,” he said.
The time to death also varies widely — from minutes to several hours. Factors such as the specific drugs used, the route of administration, and individual patient factors can all play a role. In some cases, death may occur rapidly, while in others it may take longer. This variability can be distressing for both the patient and their loved ones, particularly if they expect a swift and peaceful death, he argued.
However, the lack of reported data on complications makes the evidence as anecdotal as the evidence behind safety, Pleiter said.
The Ethical and Practical Implications
The lack of reliable data also raises concerns about informed consent. Patients are often reassured that their death will be peaceful, but without comprehensive studies, how can such promises be guaranteed?
“How can you get informed consent from a patient when the data isn’t there?” Regnard asked. “Until they produce the data, the data is purely anecdotal. We wouldn’t tolerate that level of uncertainty in palliative care, so why are we tolerating it here?”
Pleiter noted that euthanasia has been practiced in the Netherlands for two decades, with consistent guidelines that have undergone only minor revisions. More than 100,000 patients have undergone the procedure using these established protocols. The core drug dosages have remained mostly unchanged. “When the correct drugs are administered at the right doses, there are no issues, and the outcome is always certain,” he said. Having overseen almost 5000 cases, Pleiter said he has never encountered complications.
Riccio said, “Even with precautions, the process is not always smooth. There can be moments of discomfort and unexpected reactions — things we simply cannot control. But for someone whose suffering is so excruciating that he is determined to die, minor complications are completely surmountable.”
Pleiter, Regnard, and Riccio reported no relevant financial relationships.
Manuela Callari is a freelance science journalist specializing in human and planetary health. Her words have been published in The Medical Republic, Rare Disease Advisor, The Guardian, MIT Technology Review, and others.
Source link : https://www.medscape.com/viewarticle/do-we-know-enough-about-assisted-dying-drugs-2025a100064q?src=rss
Author :
Publish date : 2025-03-13 11:36:00
Copyright for syndicated content belongs to the linked Source.