TOPLINE:
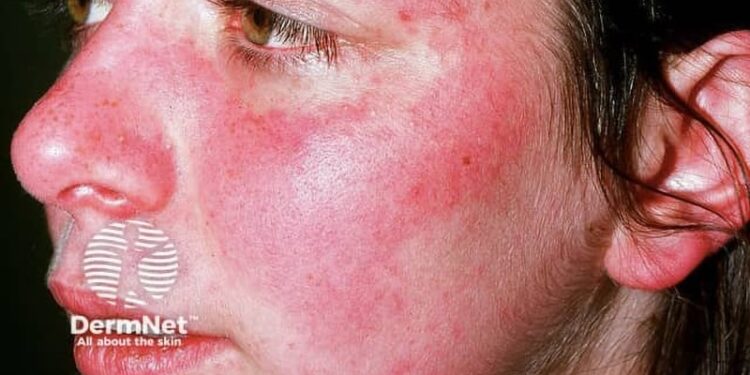
An oral therapy targeting toll-like receptors (TLR) 7 and 8 reduced disease activity in patients with cutaneous lupus erythematosus (CLE) or systemic lupus erythematosus (SLE) with active lupus rash.
METHODOLOGY:
- Cohort A of the phase 2 WILLOW study included patients with CLE and patients with mild SLE and active skin manifestation for whom no approved treatments currently exist.
- In the study of the oral, small molecule TLR 7/8 inhibitor enpatoran, researchers randomly assigned 100 patients, all receiving standard-of-care medications, to placebo, 25 mg enpatoran, 50 mg enpatoran, or 100 mg enpatoran, administered twice daily for 24 weeks.
- The primary endpoint was percent change in CL Disease Area and Severity Index Activity (CLASI-A) from baseline to week 16.
TAKEAWAY:
- Patients taking enpatoran all had clinically meaningful reductions to CLASI-A at week 16, with reductions of 74.6% for the group taking 25 mg twice daily, 59.8% for those taking 50 mg twice daily, 68.5% for those taking 100 mg twice daily, and 41.2% for those taking placebo.
- A total of 87% of patients taking 25 mg twice daily achieved ≥ 50% reduction in CLASI-A by week 24 compared with 72% for those taking 50 mg twice daily, 73.1% for those taking 100 mg twice daily, and 30.8% for those taking placebo.
- All patients on enpatoran also had reduced interferon gene signatures by week 2 compared with placebo, and this effect was continued through week 24.
- Enpatoran was well-tolerated with no new safety signals from previous clinical studies. Treatment-emergent adverse events occurred in 46% with placebo and at rates of 57.7%-80.8% in enpatoran treatment groups.
IN PRACTICE:
“The new findings from WILLOW provide promising evidence that enpatoran could enhance treatment options for these patients, addressing the suboptimal care typically available for those with CLE- and SLE-related skin manifestations,” said a spokesperson for EMD Serono, the healthcare business of Merck KGaA in the United States.
SOURCE:
The principal investigator Eric Morand, MD, of Monash University, Melbourne, Australia, presented the study at the 16th International Congress on Systemic Lupus Erythematosus in Toronto, Ontario, Canada.
LIMITATIONS:
The relatively small sample size of the trial and short duration limited the ability to detect clinically meaningful differences in efficacy and longer-term outcomes.
DISCLOSURES:
Merck KGaA, based in Darmstadt, Germany, funded the research. Morand reported financial relationships with 17 pharmaceutical companies, several of which manufacture lupus drugs.
Source link : https://www.medscape.com/viewarticle/enpatoran-shows-promise-treating-lupus-rash-2025a1000ey5?src=rss
Author :
Publish date : 2025-06-03 10:26:00
Copyright for syndicated content belongs to the linked Source.