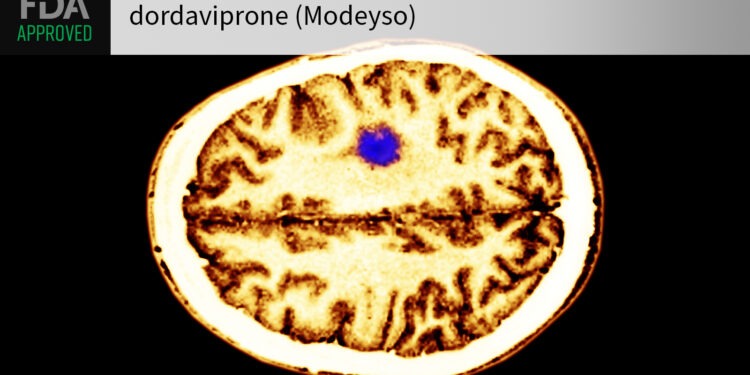
 (MedPage Today) — The FDA granted accelerated approval to dordaviprone (Modeyso) as the first systemic therapy for adults and children with diffuse midline glioma harboring H3 K27M mutations.
(MedPage Today) — The FDA granted accelerated approval to dordaviprone (Modeyso) as the first systemic therapy for adults and children with diffuse midline glioma harboring H3 K27M mutations.
Labeling stipulates use in individuals 1 year and…
Source link : https://www.medpagetoday.com/hematologyoncology/braincancer/116869
Author :
Publish date : 2025-08-06 21:20:00
Copyright for syndicated content belongs to the linked Source.