Feb. 25, 2025 — The FDA has approved a new treatment plan for a long-acting injection, making it easier for people with moderate to severe opioid use disorder (OUD) to start and keep up with their treatment.
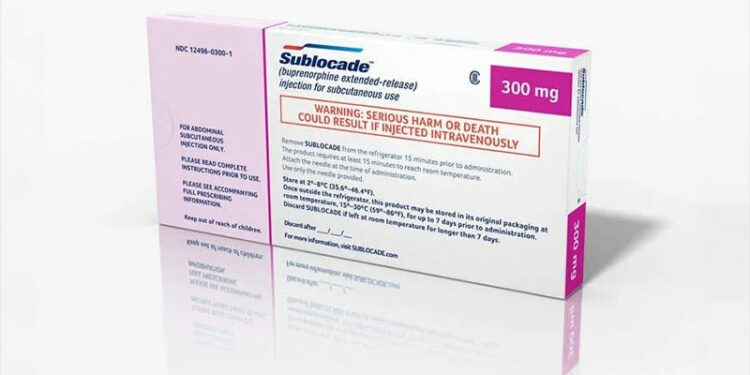
The FDA has approved an updated treatment protocol for a drug called Sublocade, making it easier for people with moderate to severe OUD to begin and continue their therapy.
The new extended clearance for Sublocade lets people get the injection after just one dose of buprenorphine (taken under the tongue or inside the cheek) and a 1-hour wait to make sure it’s safe. This means treatment can start faster and gives more options for where the shot can be given, like the stomach, thigh, buttock, or upper arm, according to the drug’s maker, Indivior.
OUD is a long-term misuse of strong painkillers (opioids) that harms a person’s physical, emotional, and mental well-being. It mostly happens when people continue taking either higher-than-prescribed or more frequent doses of opioid painkillers, or they use opioids to get high. OUD disrupts daily life significantly, leading to physical dependence, distress, and severe withdrawal symptoms when the drug is stopped. It also impacts the areas of the brain responsible for the body’s vital functions, making it a critical health concern.
Sublocade is a buprenorphine-containing long-acting injectable medicine that reduces cravings and withdrawal by partially triggering opioid receptors and preventing stronger opioids from attaching to them. First approved in 2002, this once-monthly shot under the skin starts after a week of treatment with transmucosal buprenorphine. Once injected, it forms a gel under the skin that steadily releases buprenorphine throughout the month, maintaining stable levels without daily highs and lows. Sublocade is available only through a restricted program called the SUBLOCADE REMS Program in REM-certified healthcare settings and pharmacies.
A study looked at two ways to start treatment with a long-acting opioid use disorder injection in 729 people who had used opioids for about 15 years. One group got the shot within an hour of taking a single dose of buprenorphine, while the other group waited seven days after taking daily doses. The study found that more people in the fast-start group (66.4%) got their second shot, compared to the other group (54.5%), suggesting that starting treatment sooner might help more people stick with it.
Common side effects include constipation; nausea; headache; fatigue; discomfort where the needle went in, such as pain or itching; and higher liver enzyme levels.
The FDA warns against using the injection intravenously as it can be life-threatening. Before starting treatment, patients should inform their doctor about any history of addiction, breathing problems, liver issues, or adrenal conditions and about any medications they take, especially sedatives, as taking them while on Sublocade can be dangerous. Pregnant people or those planning pregnancy should discuss the risk of opioid withdrawal in newborns. Patients should watch for reactions where they get the shot, like redness, swelling, abscesses, sores, or tissue damage, and report any unusual symptoms. Patients are advised to see their doctors before stopping Sublocade suddenly, as this can cause severe withdrawal symptoms.
SOURCES:
Indivior: “Indivior Announces FDA Approval of Label Changes for SUBLOCADE (Buprenorphine Extended-Release) Injection.”
FDA: “SUBLOCADE (Buprenorphine Extended‐Release) Injection, For Subcutaneous Use.”
Source link : https://www.medscape.com/s/viewarticle/fda-approves-new-label-opioid-use-disorder-drug-2025a10004vs?src=rss
Author :
Publish date : 2025-02-26 05:49:26
Copyright for syndicated content belongs to the linked Source.