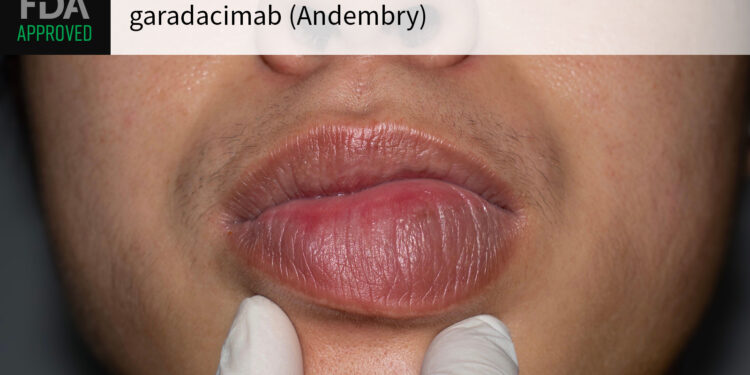
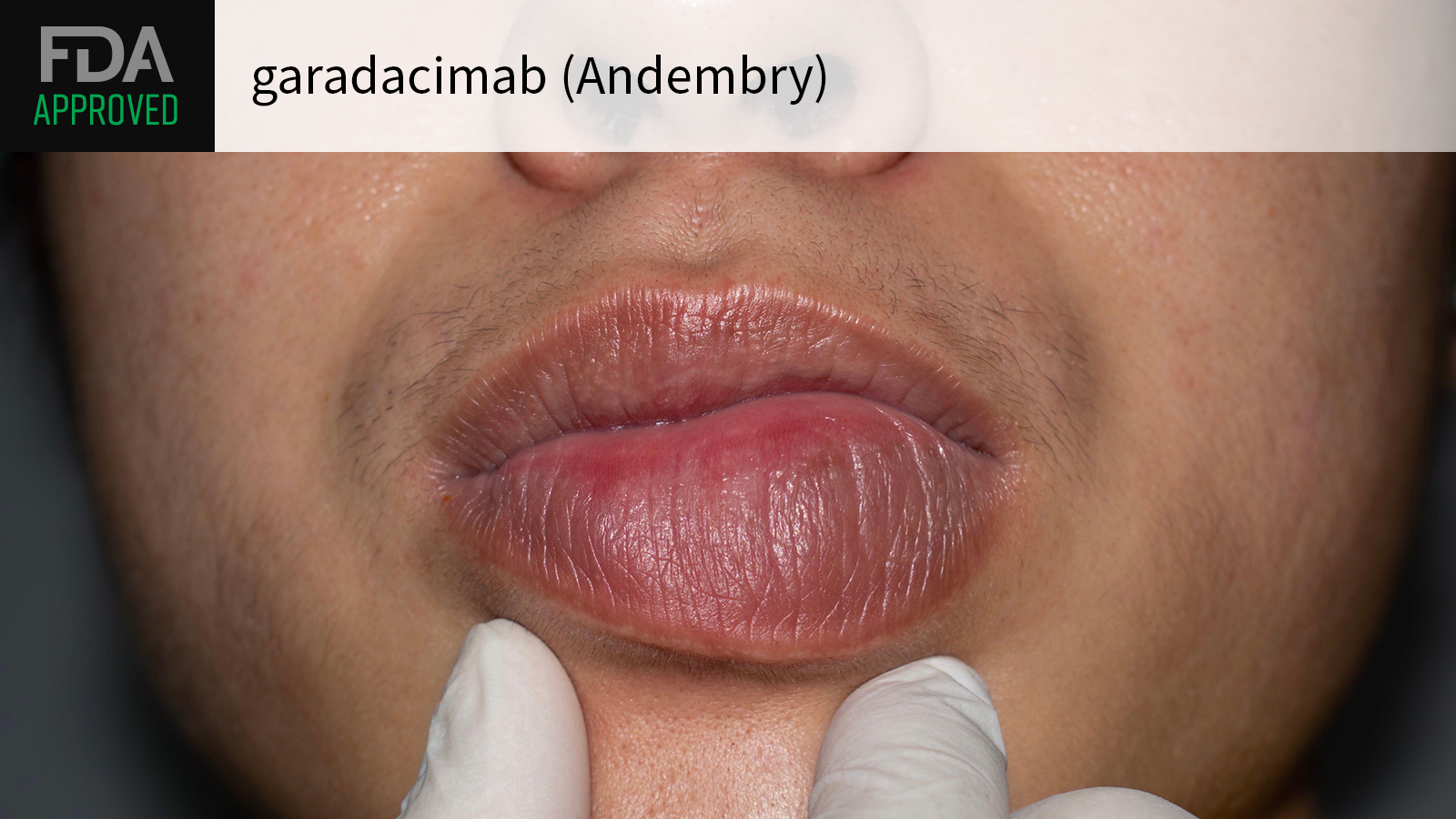
 (MedPage Today) — Anti-factor XIIa biologic garadacimab (Andembry) got the FDA greenlight for use in preventing hereditary angioedema (HAE) attacks in adult and pediatric patients ages 12 years and older, CSL announced.
(MedPage Today) — Anti-factor XIIa biologic garadacimab (Andembry) got the FDA greenlight for use in preventing hereditary angioedema (HAE) attacks in adult and pediatric patients ages 12 years and older, CSL announced.
Garadacimab is the only…
Source link : https://www.medpagetoday.com/washington-watch/fdageneral/116122
Author :
Publish date : 2025-06-17 20:32:00
Copyright for syndicated content belongs to the linked Source.