The US Food and Drug Administration has approved vimseltinib (Romvimza, Deciphera Pharmaceuticals, LLC) to treat adult patients with symptomatic tenosynovial giant cell tumors (TGCT) who will not benefit from surgical resection.
The colony-stimulating factor 1 receptor (CSF1R) blocker joins another one already on the US market for the indication, pexidartinib (Turalio, Daiichi), approved in 2019. Merck has a third CSF1 receptor blocker in development.
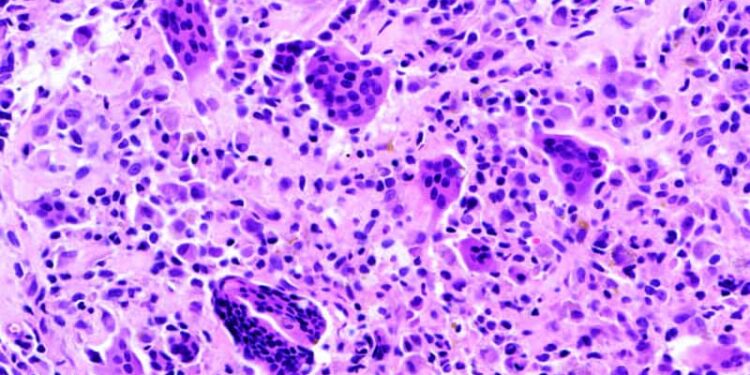
TGCT is a nonmalignant tumor that develops in the synovial membrane of joints, bursae, and tendons. The annual US incidence is about one to two cases per million people. Surgery is the primary treatment.
The condition is caused by dysregulation of the CSF1 gene with subsequent overproduction of CSF1, leading to accumulation of CSF1-positive macrophages in the synovium. Vimseltinib and pexidartinib block CSF1 signaling.
Vimseltinib was approved on the basis of the MOTION trial in 123 patients for whom surgery could lead to worse joint function or severe morbidity. Patients were randomly assigned 2:1 to vimseltinib 30 mg twice weekly or to placebo for 24 weeks.
At 25 weeks, the objective response rate was 40% in the vimseltinib arm and 0% (no responses) in the placebo arm. Median duration of response was not reached in the vimseltinib arm. After an additional 6 months of follow-up, 85% of responders (28 patients) had a duration of response of 6 months or longer, and 58% had a duration of response of 9 months or longer.
At 25 weeks, patients receiving vimseltinib also demonstrated significant improvements in active range of motion, physical functioning, and pain.
Treatment-emergent adverse events in MOTION were mostly grade 1 or 2. The most common adverse reactions (≥ 20%) included increased aspartate aminotransferase, periorbital edema, fatigue, rash, and cholesterol. There was no evidence of liver toxicity or injury in the trial, whereas pexidartinib’s label warns of potentially fatal liver injury.
M. Alexander Otto is a physician assistant with a master’s degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape. Alex is also an MIT Knight Science Journalism fellow. Email: [email protected].
Source link : https://www.medscape.com/viewarticle/fda-approves-vimseltinib-tenosynovial-giant-cell-tumors-2025a10003zt?src=rss
Author :
Publish date : 2025-02-14 21:47:35
Copyright for syndicated content belongs to the linked Source.