[ad_1]
Approval stipulates use in patients with…
[ad_2]
Source link : https://www.medpagetoday.com/hematologyoncology/breastcancer/113982
Author :
Publish date : 2025-01-28 20:41:44
Copyright for syndicated content belongs to the linked Source.
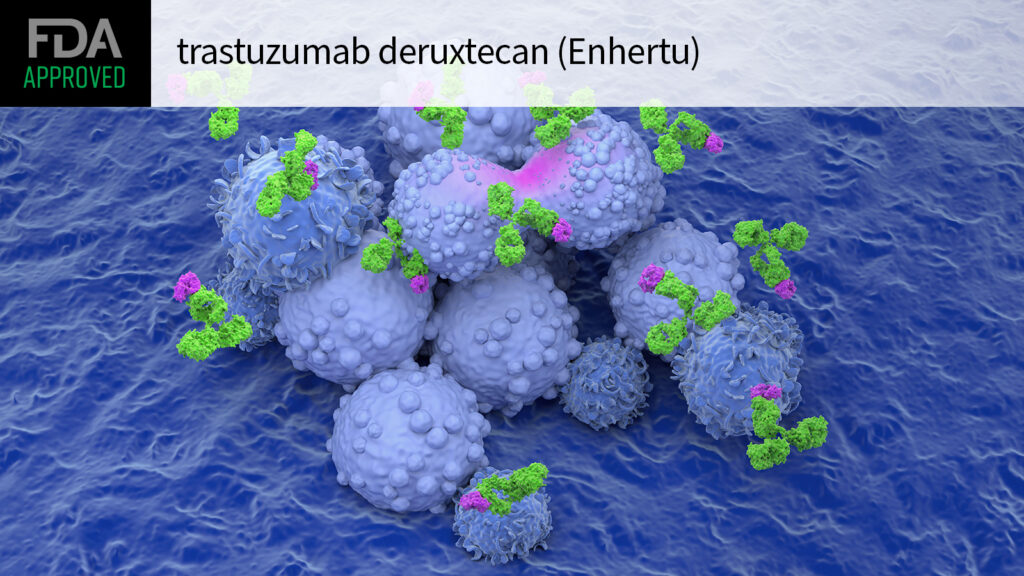
FDA Expands Enhertu Approval to HER2-Ultralow Breast Cancer