March 7, 2025 — The FDA has approved a new gene therapy for macular telangiectasia type 2 (MacTel), a rare eye disease that causes vision loss. The approval marks the first and only implantable therapy that uses cell technology to deliver a protein that helps slow the disease’s progression.
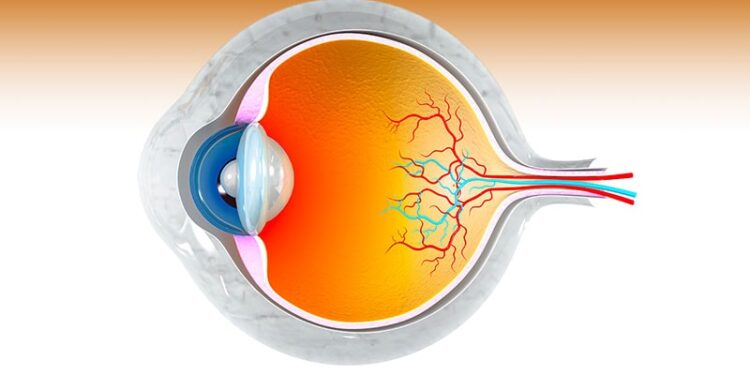
MacTel is a slowly progressing eye disease that damages the macula, the part of the eye responsible for sharp central vision needed for everyday tasks like reading, writing, driving, and recognizing faces. Over time, the light-sensing cells in both eyes break down, and the blood vessels supplying the macula become swollen and enlarged, leading to gradual vision loss.
Branded as Encelto, revakinagene taroretcel is a tiny implant containing lab-grown retinal cells. It is surgically placed in the eye, where it continuously delivers ciliary neurotrophic factor (CNTF), a protein that helps slow cell damage and vision loss. This long-term treatment reduces the need for frequent shots or other therapies.
The approval was based on two phase III clinical trials, which showed that Encelto slowed the loss of light-sensing macular cells in patients with MacTel over 2 years, as stated in a news release by Neurotech Pharmaceuticals, Encelto’s maker. The implant is expected to be available in the United States by June.
Common side effects include red or itchy eyes, bleeding or swelling inside the eyes, trouble seeing in the dark, eye pain, dry or irritated eyes, issues with stitches, small pupil size, blurry vision, headaches, worsening or new cataracts, floating spots, eye discharge, and inflammation.
Before receiving Encelto, patients should tell their doctor about any eye infections they have. If they have any eye discomfort or vision loss after getting the implant, they should seek medical care right away.
SOURCE:
Neurotech Pharmaceuticals: “Neurotech’s ENCELTO (Revakinagene Taroretcel-lwey) Approved by the FDA for the Treatment of Macular Telangiectasia Type 2 (MacTel).”
Source link : https://www.medscape.com/s/viewarticle/fda-oks-first-gene-therapy-implant-rare-eye-disease-2025a10005r0?src=rss
Author :
Publish date : 2025-03-10 04:40:00
Copyright for syndicated content belongs to the linked Source.