March 11, 2025 — The FDA has approved a new treatment for children with generalized myasthenia gravis (gMG), a disease that makes muscles weak and tired quickly. This is the first and only medicine for children 6 and older with gMG who have certain antibodies linked to the condition.
Generalized myasthenia gravis (gMG) happens when the immune system mistakenly attacks the places where nerves and muscles connect. This leads to the buildup of harmful antibodies, which overstimulate the immune system, damage cells, and make it harder for muscles to work properly.
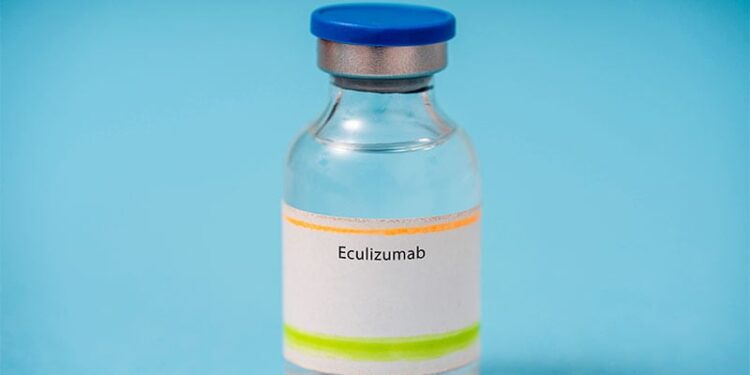
The drug, known as eculizumab but sold as Soliris, is a monoclonal antibody that blocks a specific complement protein to prevent neuromuscular damage and help relieve symptoms. First approved in 2007, the drug is already used for adults with gMG and other conditions, including blood and immune system disorders.
“This approval represents a major advancement in the treatment of pediatric myasthenia gravis and provides hope to families navigating this complex disease,” said Sharon Hesterlee, PhD, chief research officer at the Muscular Dystrophy Association, in a news release. “The availability of Soliris for children underscores the importance of continued research and innovation in neuromuscular disease treatments.”
Soliris is given through an IV drip that takes about 35 minutes for adults and 1-4 hours for children. It is available only through a special safety program (REMS) as it can increase the risk for meningococcal infections. Patients must get a meningococcal vaccine at least 2 weeks before starting treatment.
The FDA’s decision was based on clinical trial results from adults with gMG and safety studies in children, according to the release. In a 26-week study involving children aged 12-17, side effects were similar to those seen in adults, with bone and muscle pain being the most common.
SOURCE:
Muscular Dystrophy Association: “FDA Approves Expanded Use of Eculizumab (Soliris) for Pediatric Myasthenia Gravis, Bringing New Treatment Options for Children.”
Source link : https://www.medscape.com/s/viewarticle/fda-oks-first-myasthenia-gravis-treatment-children-2025a100060p?src=rss
Author :
Publish date : 2025-03-12 11:17:00
Copyright for syndicated content belongs to the linked Source.