March 21, 2025 — The FDA has approved a drug as the first and only treatment for adults with C3 glomerulopathy (C3G) to reduce protein in the urine. Unlike previous options that focused on symptom management, the approval marks the first treatment that targets the cause of the disease.
C3G happens when an overactive immune system causes a protein called C3 to build up in the kidneys. This leads to inflammation, which can damage the kidneys over time. Symptoms may include protein or blood in the urine, swelling, and, in severe cases, kidney failure that may require dialysis or a transplant. C3G often affects young people, impacting their health and daily life. While current treatments help manage symptoms, there’s a need for medications that target the root cause.
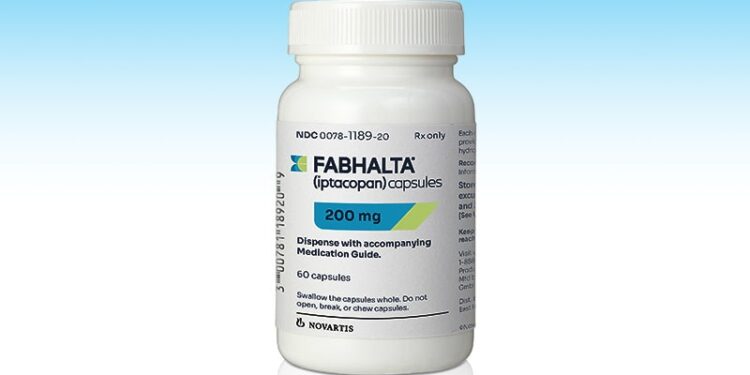
The drug, called iptacopan but sold as Fabhalta, works by blocking factor B in the immune system, reducing C3 buildup and inflammation. Fabhalta was first approved by the FDA in December 2023 for certain immune-related diseases. It is now approved to reduce proteinuria — protein in the urine — in patients with C3G, according to a news release by Novartis, the drug’s maker.
A yearlong clinical trial involving adult patients with C3G sought to find out how safe twice-daily oral Fabhalta would be and how well it would work. For the first 6 months, people in the trial received either Fabhalta or a placebo with supportive care. In the next 6 months, all of them received Fabhalta. Results showed that Fabhalta significantly reduced proteinuria within 2 weeks, with effects lasting for a year. Those who switched to Fabhalta later saw similar improvements.
Fabhalta was generally well-tolerated, with no new safety concerns reported. Common side effects include a headache, sore throat, stuffy nose, nausea, diarrhea, stomach pain, rashes, and bacterial or viral infections. Serious side effects may include increased cholesterol and triglyceride levels in the blood.
You can get Fabhalta only through a special safety program called REMS (Risk Evaluation and Mitigation Strategy). This is because the medicine can raise the risk of serious infections. To help protect against these infections, people who will take the drug need certain vaccines before starting treatment.
They must get vaccinated against Streptococcus pneumoniae (which can cause pneumonia and other infections) and Neisseria meningitidis (which can cause meningitis) at least 2 weeks before their first dose of Fabhalta.
Before taking Fabhalta, patients should tell their doctor if they have liver problems, a fever, or infections. They should also let their doctor know if they are pregnant or breastfeeding, as Fabhalta’s safety during pregnancy and breastfeeding isn’t fully known. Patients are advised not to breastfeed during treatment and for 5 days after the final dose. Patients should also tell their doctor about all the prescription and over-the-counter medications, vitamins, and herbal supplements they take, as these may interfere with Fabhalta or cause side effects. Those taking the drug should also carry a Patient Safety Card (from their doctor) with them throughout the treatment and for 2 weeks after the last dose of Fabhalta. Patients must tell their doctors right away if they have any signs of a serious infection, like a fever with shivers or chills, a headache, rashes, chest pain and coughing, cold and clammy skin, confusion, or body aches.
Sources:
Novartis: “Novartis Receives Third FDA Approval for ORAL Fabhalta (Iptacopan) — the First and Only Treatment Approved in C3 Glomerulopathy (C3G).”
Source link : https://www.medscape.com/s/viewarticle/fda-oks-first-treatment-rare-kidney-disease-2025a10006wr?src=rss
Author :
Publish date : 2025-03-24 08:49:00
Copyright for syndicated content belongs to the linked Source.