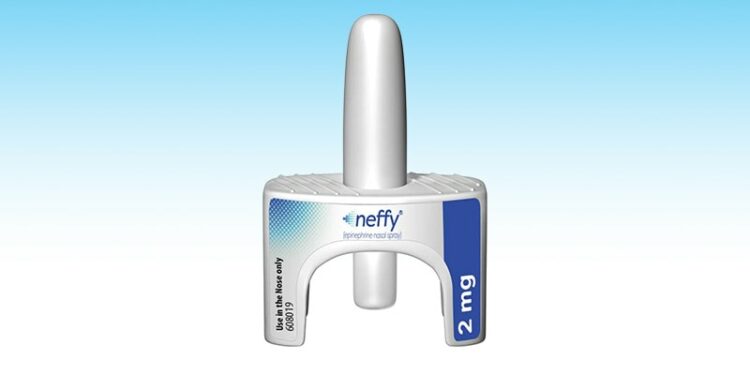
The FDA has approved a new nose spray that can quickly treat severe allergic reactions in kids ages 4 and up. This is the first emergency treatment that doesn’t use a needle. The spray, called Neffy, delivers medicine in one quick puff into the nose, making it easy to use.
Allergic reactions occur when the immune system responds abnormally to triggers like food, medication, or insect stings, causing symptoms such as hives, swelling, itching, nausea, or trouble breathing. In severe cases, a life-threatening reaction called anaphylaxis can occur within minutes and requires emergency treatment with epinephrine. This medication helps by raising blood pressure, opening airways, and reducing swelling to restore normal breathing.
First approved in 2024, Neffy 2 milligrams (nasal epinephrine) provided a needle-free option for older children and adults, while younger children under 33 pounds still had to use injection pens, which can be tricky to use and scary for kids. Now, the newly approved Neffy 1 milligram offers a safer, easier alternative, reducing delays and accidental injuries during emergencies.
The approval was based on clinical trials showing Neffy 1 milligram works as well as injectable epinephrine in children and adults, with mostly mild and temporary side effects. Neffy’s maker, ARS Pharma, stated in a press release that studies have shown that kids as young as 10 can use it correctly, and even untrained individuals like teachers and babysitters can administer it effectively.
Common side effects include nasal discomfort or tingling, nosebleeds, sneezing, runny or dry nose or throat, congestion, nausea, vomiting, headache, anxiety, dizziness, and feeling tired or overly excited.
ARS Pharma advises carrying two Neffy sprays as allergic reactions can be unpredictable, and a second dose may be needed if symptoms persist or return — using the same nostril as the first. Parents and caregivers should inform their child’s health care provider about any nasal issues, heart, kidney, or thyroid conditions, as well as any medications their child takes, since some may affect Neffy’s effectiveness. The company expects the medicine to be available by May.
SOURCE:
ARS Pharma: “ARS Pharmaceuticals Announces FDA Approval of neffy® 1 mg (epinephrine nasal spray) for Type I Allergic Reactions, Including Anaphylaxis, in Pediatric Patients Weighing 15 to < 30 Kilograms.”
Source link : https://www.medscape.com/s/viewarticle/fda-oks-nasal-spray-severe-allergic-reactions-kids-2025a10005ri?src=rss
Author :
Publish date : 2025-03-10 09:18:00
Copyright for syndicated content belongs to the linked Source.