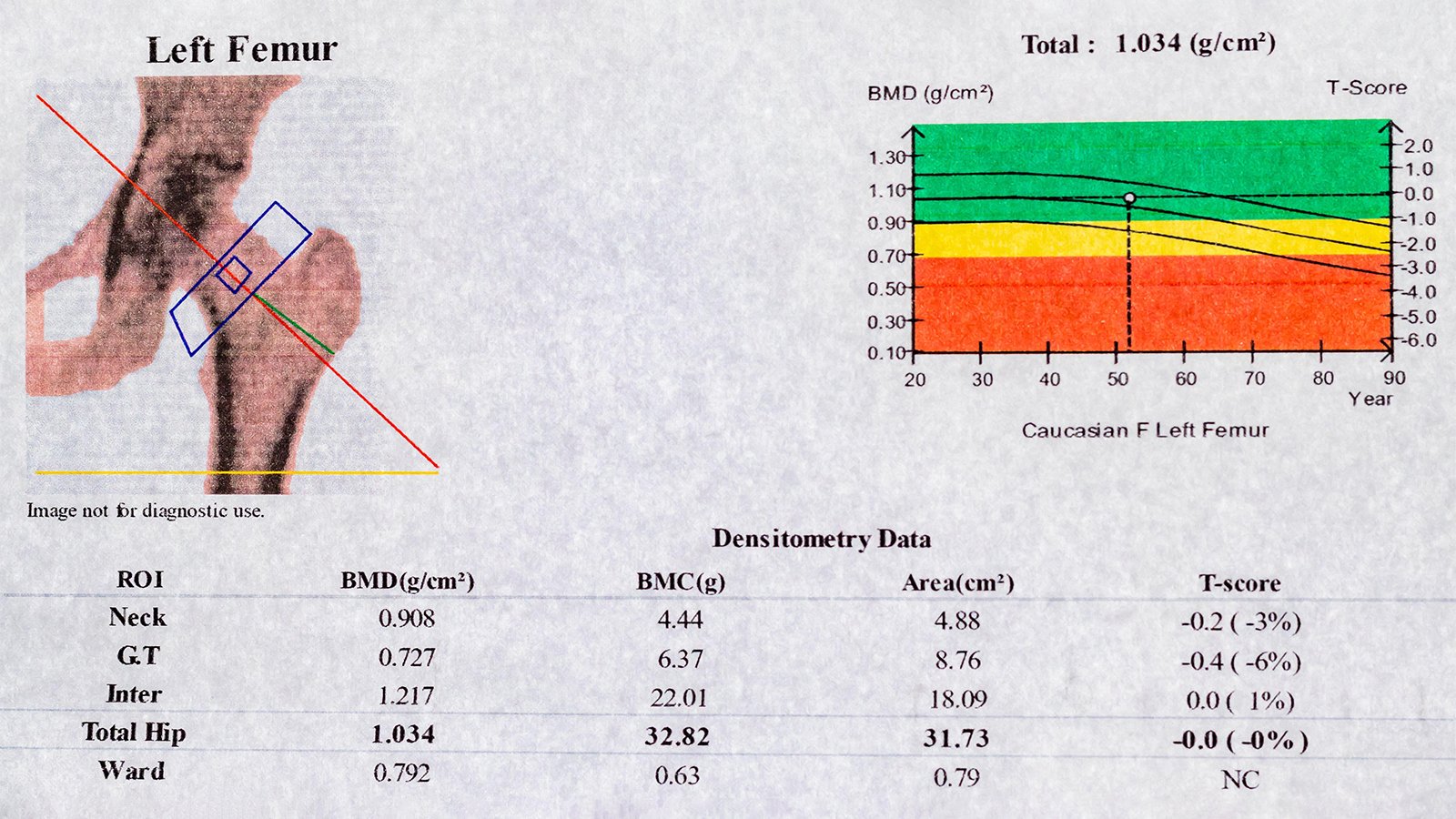
A BMD test measures calcium and other bone minerals…
Source link : https://www.medpagetoday.com/endocrinology/osteoporosis/119140
Author :
Publish date : 2025-12-22 20:19:00
Copyright for syndicated content belongs to the linked Source.
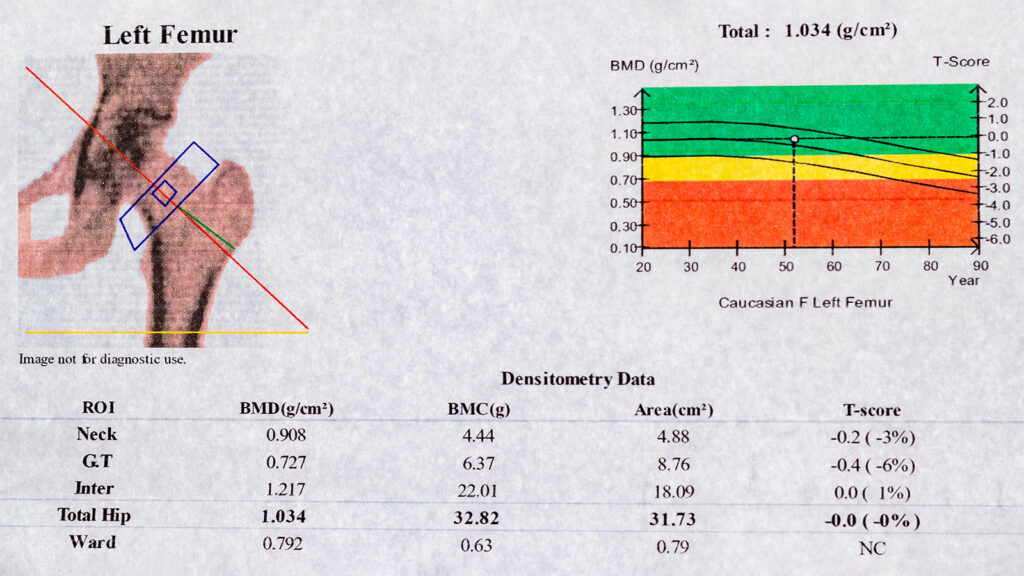

A BMD test measures calcium and other bone minerals…