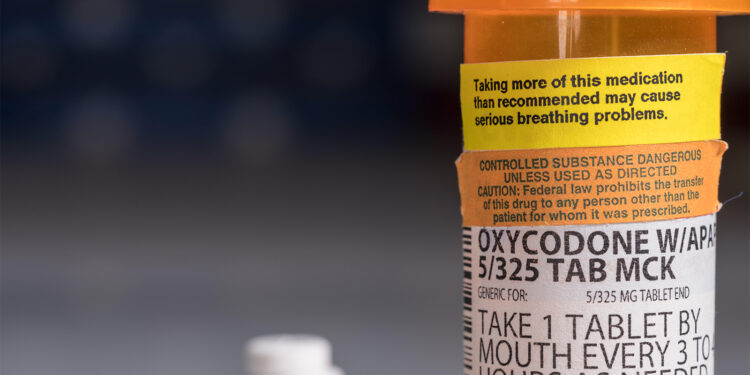
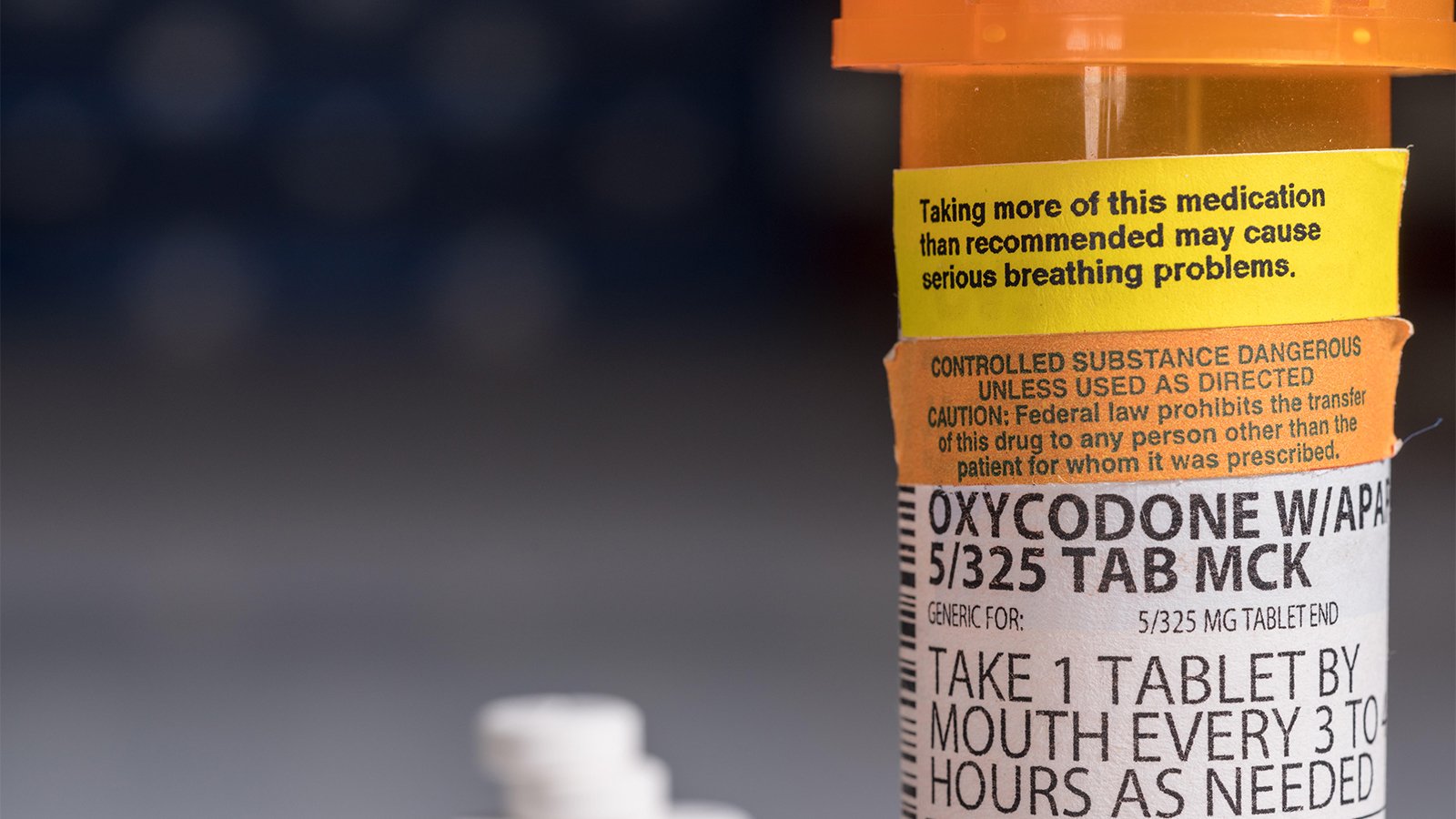
 (MedPage Today) — The FDA recently announced new requirements for opioid medications to be labeled to emphasize “the safety risks of long-term use.” The labeling updates are based on two required postmarketing observational studies on all new…
(MedPage Today) — The FDA recently announced new requirements for opioid medications to be labeled to emphasize “the safety risks of long-term use.” The labeling updates are based on two required postmarketing observational studies on all new…
Source link : https://www.medpagetoday.com/opinion/toxicology-report/117145
Author :
Publish date : 2025-08-25 19:10:00
Copyright for syndicated content belongs to the linked Source.