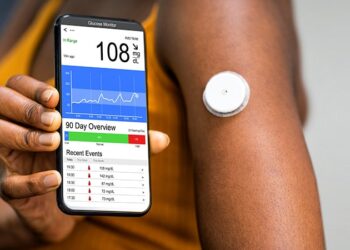
Emerging evidence points to potential benefits of continuous glucose monitoring (CGM) well beyond use as a management tool for people with diabetes who use insulin.
Until recently, CGM use had been reserved for people with type 1 diabetes (T1D) and those with type 2 diabetes (T2D) who use multiple daily doses of insulin. Even now, insurance reimbursement for others is limited. But the recent availability of over-the-counter CGMs at a cost of $75-$99 per month has led to increased CGM adoption in the United States.
At the recent Advanced Technologies & Treatments for Diabetes (ATTD) 2025 meeting held at Amsterdam, the Netherlands, data and discussions addressed the potential benefits of using CGM such as enabling behavior modification in people with non–insulin-treated T2D and prediabetes, use as a diagnostic tool for people at risk for T2D or T1D, and cost-effectiveness.
An upcoming consensus statement from ATTD, the International Diabetes Federation, and other diabetes professional organizations is expected to address uses of the technology in people with T2D or who are at risk, along the lines of this open-access review paper. It lays out evidence and provides recommendations for CGM use in people with normoglycemic obesity, intermediate hyperglycemia (a term now preferred by some instead of “prediabetes”), and in subgroups of those with T2D. The paper also includes a section about CGM use in primary care, where most of these patient groups are managed.
“Now it will be used for diagnostics, as a means to intervene early. The biggest cost savings will be in early detection and early interventions, hopefully to prevent type 2 diabetes, and with it all the complications,” lead author and conference speaker Tadej Battelino, MD, head of the Department of Pediatric and Adolescent Endocrinology at University Medical Center Ljubljana, Ljubljana, Slovenia, told Medscape Medical News.
“In 5 years, nobody will use anything else…All the other approaches will be forgotten,” Battelino added.
Regarding CGM use in those with T2D, conference speaker Irl B. Hirsch, MD, medical director of the Diabetes Care Center of the University of Washington Medical Center, Seattle, said “In my opinion, adding the sensor is no different than adding another drug for type 2 diabetes, with as good, if not better, response than some of the drugs. The reason is that people are making lifestyle decisions based on the glucose levels. It’s a very powerful behavioral modification that improves overall health.”
However, it will likely take time for CGM to reach everyone who can benefit from it, in part due to affordability but for other reasons as well.
David T. Ahn, MD, chief of Diabetes Services at Hoag Memorial Hospital Presbyterian, Newport Beach, California, told Medscape Medical News that he offers CGM to all his patients with T2D but that those who accept “tend to self-select for those that are interested in harnessing the data and ‘learning about their body’…Many people who are not on insulin may not require long-term, continuous usage of CGM, and instead may stop using it after an initial 6- to 12-week learning period or transition over to an intermittent use scenario.”
Diagnosing Diabetes: An Evolving Picture
Viral N. Shah, MD, professor of endocrinology and director of diabetes clinical research at Indiana University School of Medicine, Indianapolis, spoke about CGM use in prediabetes and diabetes diagnosis. Two over-the-counter CGMs are currently on the US market that could be used for this purpose, Dexcom’s Stelo and Abbott’s Lingo (Abbott’s Rio CGM is for those with non–insulin-treated T2D).
The thinking on this is evolving, Shah noted. “Can we use CGM to replace fasting plasma glucose, the 2-hour oral glucose tolerance test [OGTT], or A1c for diagnosing prediabetes? I don’t think we are there yet, but the research is in progression… I think the first target is to get rid of OGTT if possible. It’s cumbersome, and people don’t like to do it…A lot of research is looking at CGM-based criteria.”
He pointed to an article he co-authored titled “CGM for prediabetes: What are the best metrics?” It includes a literature review and some preliminary consensus conclusions. For one, the “time in range” target of 70-180 mg/dL used for people with diabetes isn’t appropriate for those with prediabetes as the goal is preventing progression to T2D and 140 mg/dL is the upper limit of normal for people without diabetes. The lower limit may also need to be adjusted downward. And rather than a goal of 70% time in range, in prediabetes the authors proposed a target of 95%-98% of the time spent in the 54-140 mg/dL range.
Moreover, some type of CGM-based glycemic variability metric will likely also be needed as identifiers of early dysglycemia. In particular, the mean amplitude of glycemic excursion (MAGE) appears to be particularly predictive of dysglycemic progression. And as people with prediabetes rarely experience hypoglycemia, that metric is likely to be de-emphasized.
“We still don’t know the best metrics to use, but now more evidence is coming out,” Shaw noted.
Ahn, who participated in some of the discussions around these new CGM uses, told Medscape Medical News, “I think there is still not a clear path forward to using CGM for the diagnosis of T2D. Among a panel of 18 expert diabetes clinicians, there was no clear consensus about which CGM tracings from a nondiabetes population warranted further evaluation or discussion with a clinician. So while the concept of being able to diagnose with CGM sounds appealing, defining what makes a CGM abnormal or normal is not straightforward at the moment.”
The path may be clearer for use in pre-T1D, Chantal Mathieu, MD, PhD, an endocrinologist at the Katholieke University, Leuven, Belgium, noted there are now ICD-10 codes (E10, A0-A2) for preclinical T1D based on autoantibody screening. For those with stage 2 (E10, A2), where beta cell mass is significantly reduced, “the challenge is that we don’t know for an individual when the progression to stage 3 T1D will happen,” she said.
Here again, data suggest that levels above an upper cutoff of 140 mg/dL predict progression to clinical (stage 3) T1D, and thus can be a useful tool to follow these individuals, she noted.
Cost Considerations: CGM for All, or Just Those Who Can Afford It?
Cost and access remain barriers to CGM use. Currently in the United States, the devices are typically only covered by insurance for people with diabetes who use insulin or are otherwise at risk for hypoglycemia, although sometimes patients can get the prescribed CGMs at a lower cost than over-the-counter versions using coupons, Hirsch noted.
According to Ahn, “As of now, not all commercial insurance plans are covering CGM for basal insulin only users, which Medicare now covers, but the requirements for coverage are slowly loosening over time. The hope is that more and more patients — first non-insulin requiring people with diabetes, then later pre-diabetes — would have insurance coverage for CGM.”
But, in a presentation at the meeting, Hirsch said he is concerned right now that people with diabetes who are on Medicaid will lose access to CGM and to their medications if those benefits are cut. Of the 79.3 million Medicaid recipients in the United States, 13%, or 10 million, have diabetes. Of those, about 25%-30%, or 2.5-3 million, use insulin and therefore would qualify for CGM coverage. The federal government funds 50%-74% of Medicaid, with the rest funded by states.
“It’s the highest line item in the state’s budget. Here in Washington State, which is $4 billion in debt, we are already seeing it more difficult to get sensors and pumps,” he told Medscape Medical News.
Effective for Non-Insulin Users?
As of now, most of the CGM cost effectiveness studies informing insurance coverage have been for insulin users. But at the ATTD meeting, a modeling study presented by Hamza Alshannaq, MD, of Dexcom, showed that real-time CGM was cost-effective for public payors and cost-saving for commercial payers for the non–insulin-treated population with T2D in Canada.
Roy W. Beck, MD, PhD, executive director of the Jaeb Center for Health Research, Tampa, Florida, summarized efficacy data for CGM in people with T2D not on insulin, pointing to a meta-analysis of six randomized controlled trials of CGM use in 407 non–insulin-treated people with T2D, showing improved glycemic control compared with fingerstick glucose monitoring.
He also cited numerous studies linking a higher CGM-measured time in range with lower rates of diabetes-related complications in T2D, including retinopathy, albuminuria, peripheral neuropathy, and mortality.
Beck acknowledged “we do need a larger study to provide more compelling evidence” for cost-effectiveness of CGM in non–insulin-treated T2D. However, he said, “the improved A1c and time in range, if we extrapolate that over a lifetime, will be associated with a reduced rate of chronic complications and as a consequence there will be reduced costs associated with the treatment of complications and also less loss of productivity…There’s potential for reduction in direct costs with CGM that would offset the costs of CGM itself.”
Battelino had served on advisory boards for, and/or received speakers’ bureau honoraria from Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic, Abbott, DreaMed Diabetes, Indigo Diabetes, Dexcom, and Roche. Hirsh had conducted research for Dexcom, Tandem, and Mannkind, and does consulting for Abbott, Roche, and Hagar. Shah had received research support from Alexion, Novo Nordisk, Dexcom, Enable Bioscience, Zucara Therapeutics, Lilly, Breakthrough T1D, and NIH. He serves as a consultant, speaker, and/or advisory board member for Dexcom, Insulet, Tandem, Ascensia, Embecta, Lilly, Sanofi, Novo Nordisk, Sequel Med Tech, Biomea Fusion, Lumosfit, and Genomelink. Ahn had received speaker’s, consulting, and/or advisory fees from Abbott, Ascensia, Lilly, Mannkind, Insulet, Novo, Sequel, and Xeris.
Mathieu had served on advisory boards and/or is a speaker for Novo Nordisk, Sanofi, Eli Lilly, Novartis, Dexcom, Boehringer Ingelheim, Bayer, Roche, Abbott, Medtronic, Insulet, Biomea Fusion, SAB Bio, and Vertex, with financial compensation received by her institution. Beck had no personal disclosures. His institution had received grant funding from Dexcom and Abbott Diabetes Care, and study supplies from Dexcom, Abbott, and Medtronic.
Miriam E. Tucker is a freelance journalist based in the Washington DC area. She is a regular contributor to Medscape, with other work appearing in the Washington Post, NPR’s Shots blog, and Diatribe. She is on X (formerly Twitter) @MiriamETucker and BlueSky @miriametucker.bsky.social.
Source link : https://www.medscape.com/viewarticle/continuous-glucose-monitoring-insulin-users-and-beyond-2025a10007mx?src=rss
Author :
Publish date : 2025-03-31 11:44:00
Copyright for syndicated content belongs to the linked Source.