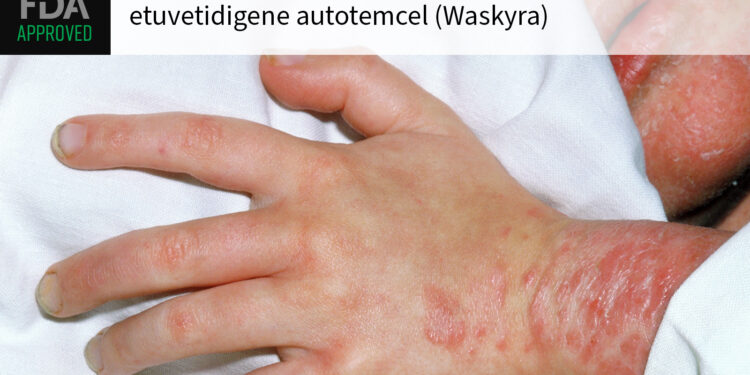
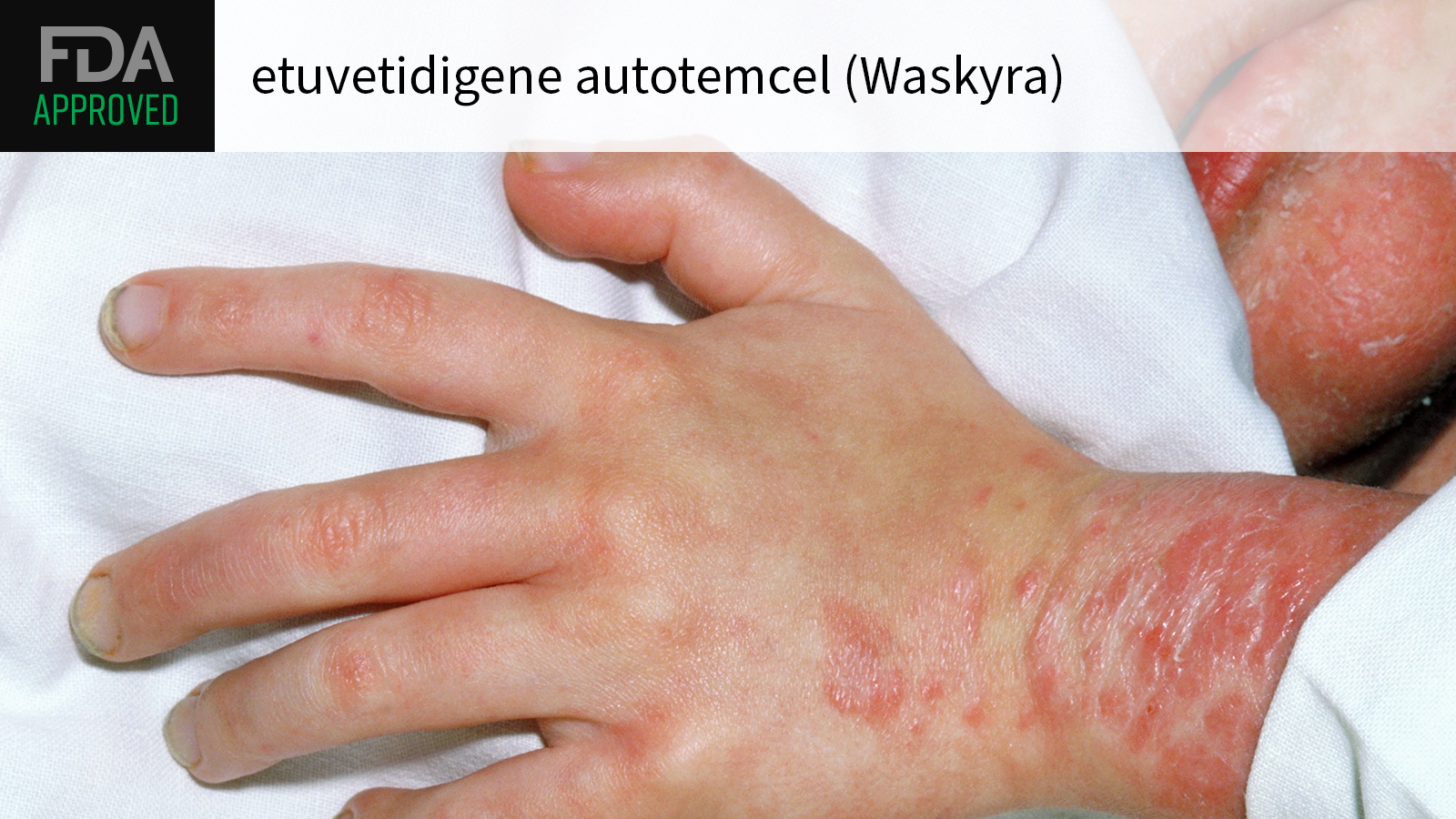
 (MedPage Today) — The FDA on Tuesday approved etuvetidigene autotemcel (Waskyra) as the first cell-based gene therapy for patients 6 months and older with Wiskott-Aldrich syndrome, a rare primary immunodeficiency.
(MedPage Today) — The FDA on Tuesday approved etuvetidigene autotemcel (Waskyra) as the first cell-based gene therapy for patients 6 months and older with Wiskott-Aldrich syndrome, a rare primary immunodeficiency.
The life-threatening genetic…
Source link : https://www.medpagetoday.com/genetics/generalgenetics/118940
Author :
Publish date : 2025-12-10 21:39:00
Copyright for syndicated content belongs to the linked Source.