TOPLINE:
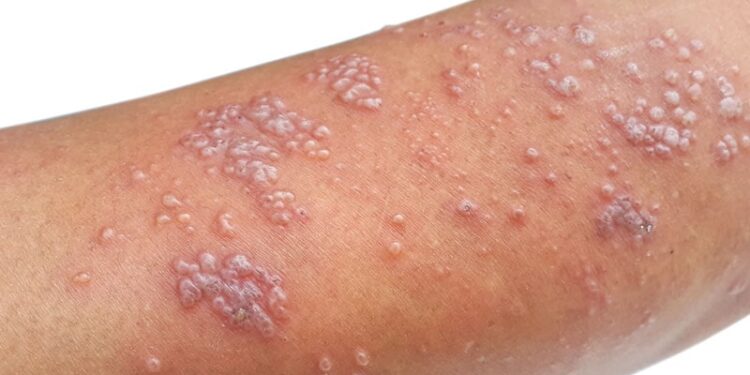
Patients with moderate to severe psoriasis treated with ustekinumab and guselkumab showed a trend towards a lower risk for herpes zoster (HZ) and postherpetic neuralgia (PHN) than those receiving traditional systemic treatments. Adalimumab was associated with a significantly higher risk for HZ.
METHODOLOGY:
- Researchers conducted a retrospective cohort study of patients with psoriasis or psoriatic arthritis from Taiwan’s National Health Insurance Research Database during 2011-2021 who were treated with biologics (6769 patients) or traditional systemic treatments (4594 patients) for at least 6 months.
- Biologics received were etanercept (n = 815), adalimumab (n = 1870), ustekinumab (n = 1095), secukinumab (n = 2327), ixekizumab (n = 261), brodalumab (n = 303), and guselkumab (n = 98). Traditional systemic treatments involved methotrexate, cyclosporine, or acitretin.
- The primary outcome was HZ infections, and the secondary outcome was subsequent PHN.
TAKEAWAY:
- Incidence rates of HZ per 1000 person-years were the highest for adalimumab (27.2) and the lowest for guselkumab (6.6).
- Patients who received adalimumab showed a significantly higher risk for HZ than those who received traditional systemic treatments (hazard ratio [HR], 1.63; 95% CI, 1.22-2.18).
- Ustekinumab (HR, 0.82; 95% CI, 0.61-1.14) and guselkumab (HR, 0.48; 95% CI, 0.22-1.02) showed trends toward a lower HZ risk.
- Ustekinumab was associated with a significant reduction in the risk for PHN compared with traditional systemic treatments (HR, 0.22; 95% CI, 0.08-0.64). There were no significant differences in HZ risk with etanercept, secukinumab, ixekizumab, or brodalumab compared with traditional systemic treatments.
IN PRACTICE:
Ustekinumab and guselkumab may be associated with a reduced risk for HZ and PHN compared with traditional systemic treatments, the authors concluded. Although not statistically significant in some comparisons, they wrote, “the trends suggest a potential protective effect…These findings could be significant for real-world practice since they underscore the need to prioritize biologics with favorable safety profiles in clinical practice.”
SOURCE:
This study was led by Chaw-Ning Lee, of the Department of Dermatology, National Cheng Kung University in Tainan, Taiwan, and was published online on March 20 in the British Journal of Dermatology.
LIMITATIONS:
Data on psoriasis severity scores and HZ vaccination rates were not available. The study excluded self-paying patients, limiting generalizability. Some confounding factors could be present as well.
DISCLOSURES:
The study was supported by the National Science and Technology Council of Taiwan and the National Health Research Institutes of Taiwan. The authors reported no conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/herpes-zoster-risk-psoriasis-biologics-vs-traditional-2025a10007g2?src=rss
Author :
Publish date : 2025-03-28 10:33:00
Copyright for syndicated content belongs to the linked Source.