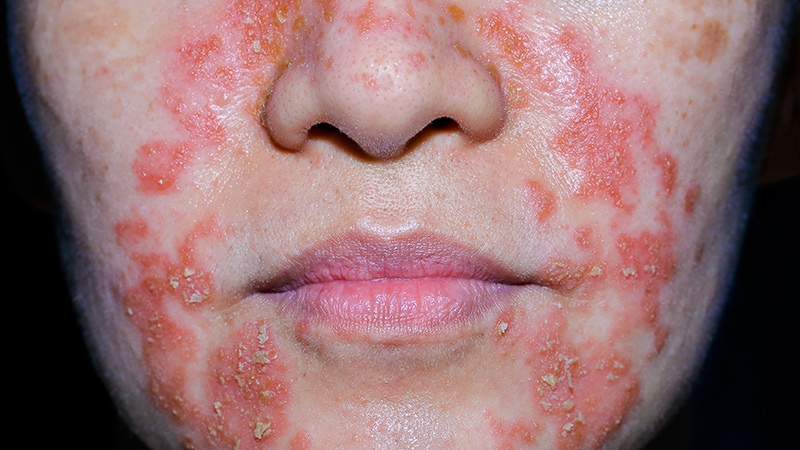
NEW YORK — Topical antifungals should no longer be considered a first-line therapy for seborrheic dermatitis for two reasons: Topical anti-inflammatories have replaced them, and rates of drug-resistant fungal infections are rising, according to an update on this skin disease.
Now that there is compelling evidence that seborrheic dermatitis is best characterized as an inflammatory disease, the use of topical antifungals, despite the efficacy they offer, should be placed further down the list of therapeutic options, according to Benjamin N. Ungar, MD, Director of the Rosacea and Seborrheic Dermatitis Clinic, Icahn School of Medicine at Mount Sinai, New York City.
In a series of warnings about treatment-resistant fungal infections from the Centers for Disease Control and Prevention, dermatologists are included among antifungal over-prescribers, he noted.
Topical Anti-Inflammatory Is First Line
In an update on seborrheic dermatitis at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024, Ungar provided evidence that anti-inflammatory therapies “directly target the pathology.”
The underlying pathophysiology of seborrheic dermatitis was once unclear, but there is now overwhelming evidence that inflammation is the driving force. Citing analyses at his institution conducted with tape strips, which can yield much of the information gained from biopsy, patients can be differentiated from controls for genetic markers of upregulated inflammation.
“The inflammatory profile is largely skewed toward Th17,” said Ungar, calling this profile “a little psoriasis-like,” even if this signature supports a distinct pathology.
In contrast, the lack of a significant upregulation of Th2 inflammatory markers rules out an eczematous-type pathology, he said.
From a clinical perspective, the best evidence that seborrheic dermatitis is driven by inflammation comes from the pivotal phase 3 trial of patients aged 9 years or older with seborrheic dermatitis that led to the approval of 0.3% roflumilast foam for seborrheic dermatitis in December 2023. A phosphodiesterase-4 inhibitor that downregulates multiple inflammatory pathways, the efficacy of this agent has been remarkable, according to Ungar.
For the relatively rigorous primary endpoint of investigator global assessment (IGA) score of 0 (clear) or 1 (almost clear), the difference was already significant at 2 weeks (43.0% vs 25.7% of those on the vehicle; P P
Seborrheic Dermatitis, Rosacea Can Occur Concomitantly
One of the persistent challenges for skin disease that looks like seborrheic dermatitis is the overlap with rosacea, Ungar said. With progress in understanding the distinct pathologies of these common skin conditions, he suggested that the clinical picture can be particularly confusing when these are concomitant and said it is important to make the distinction.
“We do not want to help seborrheic dermatitis at the cost of worsening rosacea,” he said, referring specifically to the use of topical steroids that exacerbate rosacea even if they are effective against seborrheic dermatitis.
Because of its efficacy and low relative risk for adverse events, topical roflumilast is a more appropriate first-line agent than topical steroids, but Ungar acknowledged that not all patients respond, and other options are needed.
Whereas Promiseb, a topical cream with antifungal and anti-inflammatory properties, can be effective, Ungar said other nonsteroidal anti-inflammatory therapies, such as topical calcineurin inhibitors, might be among the off-label alternatives or adjuncts to consider when first-line therapies fail.
Interleukin (IL) 12/IL-23 Inhibitor Is Promising in Seborrheic Dermatitis
In a published case series for which he served as a co-author, ustekinumab, an IL-12/IL-23 inhibitor, provided relatively durable complete or near-complete lesion resolution in patients with treatment-resistant seborrheic dermatitis, Ungar said. These data provide a clue that “there could be a role for systemic targeted therapies” in challenging cases, he added.
Because of the “more comprehensive” understanding of the pathophysiology of seborrheic dermatitis, treatments are being targeted in a more rational way, but Ungar suggested that further progress depends on better and more efficient methods for making a diagnosis, developing specific treatment strategies for patients with overlapping seborrheic dermatitis and rosacea, and identifying alternative or additional therapies for those with an inadequate response.
“Although we have excellent drugs helping a lot of people [with seborrheic dermatitis], there are still additional therapies needed, particularly in those with more serious disease involvement,” Ungar said.
Asked for his perspective, J. Mark Jackson, MD, clinical professor of dermatology, University of Louisville, Louisville, Kentucky, indicated that regulatory approval of topical roflumilast changed the therapeutic landscape for seborrheic dermatitis. Jackson was the lead author of a review article on unmet needs for seborrheic dermatitis that was published in March 2024.
Topical roflumilast “should be the first-line agent for seborrheic dermatitis due to its mechanisms of action and the inflammatory nature of the disease,” he said. “Topical antifungals, albeit helpful, do not act on the innate inflammatory process of seborrheic dermatitis, and topical steroids are not best for use on the face, if possible,” he added.
Ungar reported financial relationships with topical roflumilast manufacturers Arcutis Biotherapeutics, Bristol Myers Squibb, Castle Biosciences, Fresenius Kabi, Galderma, Janssen Pharmaceuticals, Eli Lilly and Company, Pfizer, Primus, Sanofi, and UCB. Jackson reported financial relationships with AbbVie, Arcutis Biotherapeutics, Bristol Myers Squibb, Dermavant, Evommune, Janssen Pharmaceuticals, Eli Lilly and Company, Novartis, Pfizer, Regeneron, Sanofi, and UCB.
Ted Bosworth is a medical journalist based in New York City.
Source link : https://www.medscape.com/viewarticle/inflammation-now-key-target-seborrheic-dermatitis-2025a100005x?src=rss
Author :
Publish date : 2025-01-06 05:48:09
Copyright for syndicated content belongs to the linked Source.