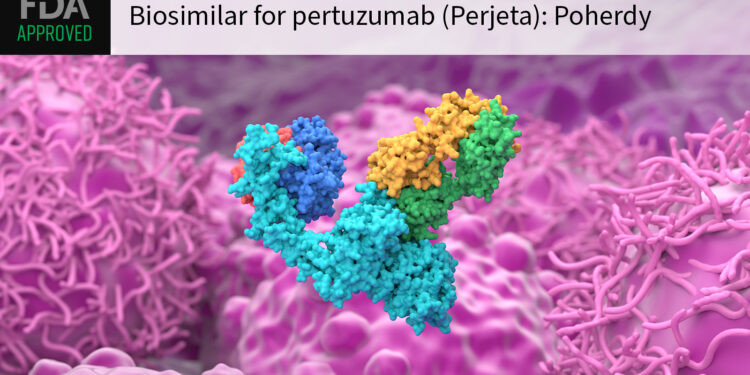
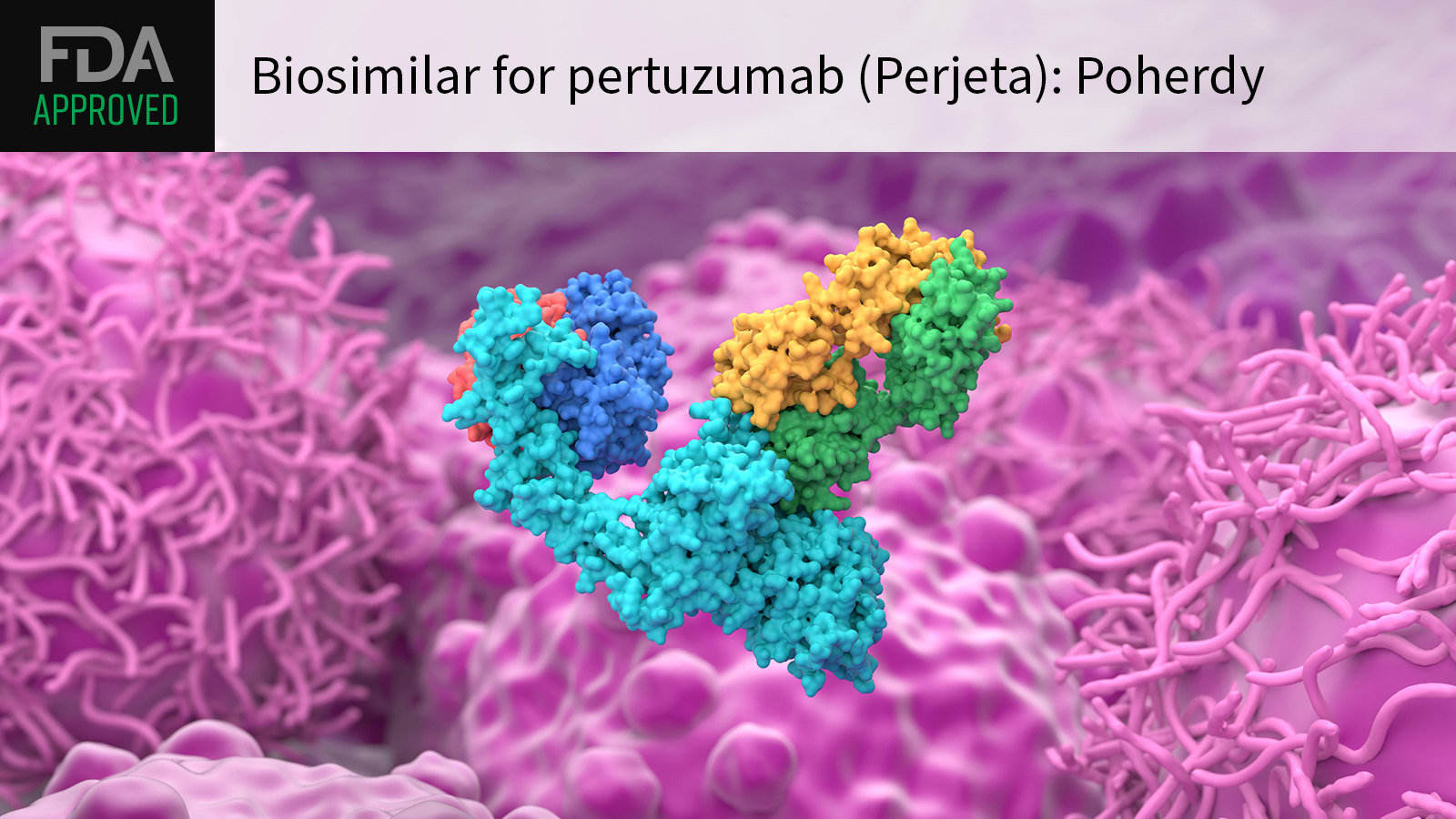
 (MedPage Today) — The FDA approved pertuzumab-dpzb (Poherdy) as the first biosimilar for its reference product Perjeta, a monoclonal antibody commonly used in standard regimens for HER2-positive breast cancer.
(MedPage Today) — The FDA approved pertuzumab-dpzb (Poherdy) as the first biosimilar for its reference product Perjeta, a monoclonal antibody commonly used in standard regimens for HER2-positive breast cancer.
FDA granted the targeted agent an…
Source link : https://www.medpagetoday.com/hematologyoncology/breastcancer/118513
Author :
Publish date : 2025-11-13 21:29:00
Copyright for syndicated content belongs to the linked Source.