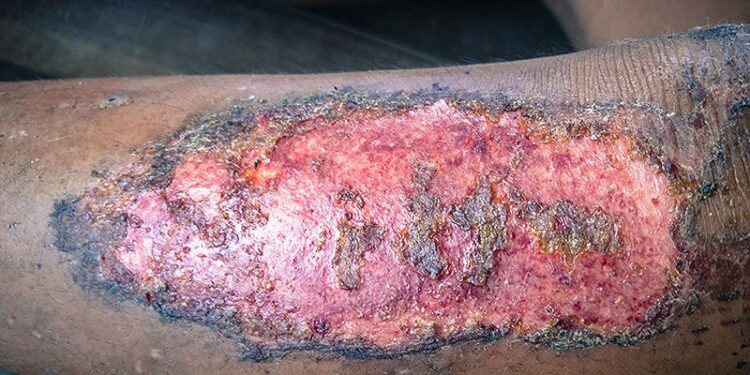
In a recently published phase 3 trial, credit card-sized cultured skin grafts corrected for the COL7A1 mutation that causes recessive dystrophic epidermolysis bullosa (RDEB) and enabled most patients to achieve at least 50% reductions in the size of large chronic wounds, with an overall mean pain score reduction of more than 2 points at week 24.
In April 2025, prademagene zamikeracel (Zevaskyn, Abeona Therapeutics) became the first FDA-approved cell-based genetic therapy when it was approved for the treatment of wounds in adult and pediatric patients with RDEB. It is the first commercially available RDEB treatment to demonstrate sustained wound healing and pain reduction for large, chronic RDEB wounds, according to investigators.
“These wounds are the most terrible and difficult to treat in our patients,” the study’s lead principal investigator Jean Y. Tang, MD , PhD, professor of dermatology at Stanford University School of Medicine in Stanford, California, said in an interview. “To have a therapy using the patient’s cells to suture on, hopefully close their wounds, and reduce their pain is monumental.”

For the VIITAL trial, published online on June 23 in The Lancet, Tang and colleagues enrolled 11 patients with clinically and genetically confirmed RDEB (median age, 21 years) and no evidence of immune response to type VII collagen. To reduce the likelihood of immunogenicity, only patients with the amino-terminal NC1 fragment of type VII collagen could enroll. Investigators selected 43 wounds of at least 6 months’ duration measuring at least 20 cm2 for treatment and compared these results against standard care for 43 randomly assigned control wounds matched for size, chronicity, and location.
Grafting Process
Using 8-mm punch biopsies from unaffected skin, investigators transduced isolated keratinocytes with a retrovirus carrying the full-length human COL7A1 gene, then used those keratinocytes to culture up to 12 40 cm2 sheets of autologous keratinocytes per patient. After 25 days, surgeons sutured up to six sheets of prademagene zamikeracel per patient, with each procedure taking 3-4 hours. To minimize pressure and friction, patients remained hospitalized with no changes of nonadhesive contact dressings for 7 days postsurgery.
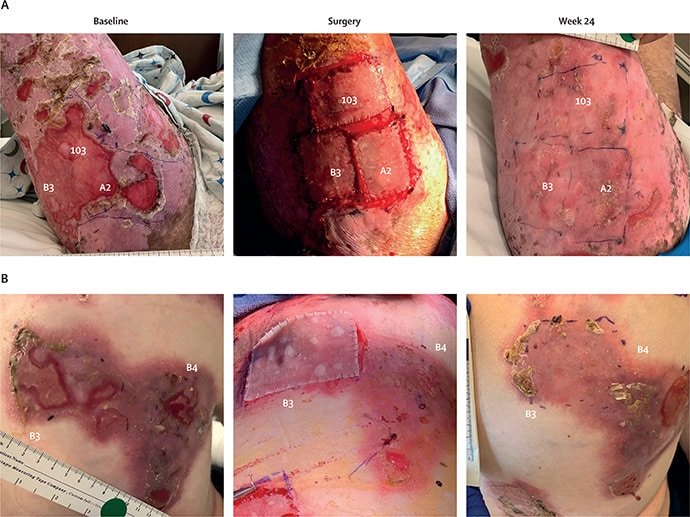
Investigator assessments showed that 24 weeks posttreatment, 81% of treated patients achieved at least 50% healing from baseline vs 16% of control wounds (P < .0001). Mean pain reduction from baseline (measured with the Wong-Baker Faces scale within 3 hours after dressing change) was 3.07 among treated patients vs 0.90 for control wounds (P = .0002). Also at week 24, 16% of treated wounds achieved complete healing, with a 2.0-point decrease in itch severity from baseline. The corresponding figures for control wounds were 0 (healing) and 0.5 (itch).
In the past 3 years, the FDA and the European Medicines Agency also have approved topical beremagene geperpavec (Vyjuvek) and birch triterpenes (Filsuvez) for dystrophic EB. However, wrote Tang and colleagues, the wounds treated with these therapies were mostly less than 20 cm2, and both treatments require repeated application. Nor did they improve pain or itchin clinical trials, added Tang.
Having the first permanent gene correction for RDEB is very exciting, said Amy Paller, MS, MD, professor and chair of Dermatology and professor of pediatrics at Northwestern University, Chicago. She was not involved with the phase 3 study but will run the first of several specialized centers where prademagene zamikeracel will be applied.

“This is the first instance in our field where a gene has been corrected for grafting and is commercially available,” Paller said. “It’s something that we and our patients dreamed about for genetic skin disorders.”
Logistics and Labor
Performing the treatment is logistically complex and “incredibly labor-intensive,” Paller said. The process requires rushing biopsies to Abeona’s good manufacturing practice facility in Cleveland, where over the next few weeks, the keratinocytes are grown out, corrected, expanded markedly, and quality tested. “It’s a very expensive procedure with many moving parts,” she said.
Accordingly, Paller plans to start with three patients from her own practice, beginning in August. Additionally, she is consulting with other interested families in the Midwest and will soon expand outreach to her other patients. “I want experience with the process in patients I have known for years before grafting additional patients,” she explained.
Prademagene zamikeracel’s retroviral component may provoke discussion. Tang explained, “We take the biopsy from the patient’s skin, grow their keratinocyte skin cells, and use a retrovirus containing wild-type collagen VII to introduce that into the patient’s skin cells. There’s always a theoretical concern of retroviruses maybe hitting off-target genes, but so far, we and others haven’t seen that.” In a phase 1/2a study, investigators followed seven patients treated with what was then known as EB-101 for a mean of 5.9 years. There were no serious adverse events related to treatment, with no gene therapy-related cutaneous or extracutaneous malignancies or evidence of systemic replication-competent retrovirus infections in serum samples from patients.
The beauty of grafting skin, Paller added, is that development of a tumor — while unexpected — would be easily visible and biopsied, just as dermatologists now biopsy for suspected squamous cell carcinoma, a feared complication related to the scarred skin in patients with RDEB. Treated patients will require a long-term commitment to surveillance, she said, with a low threshold for considering biopsy if a change suggesting carcinoma is seen. The FDA recommends that manufacturers of genetic products follow patients for 15 years posttreatment.
Clinical and Research Implications
Although the phase 3 study showed the utility of correcting genetically defective collagen VII in treating RDEB, said Tang, the cell therapy approach could prove useful for additional genetic skin diseases such as ichthyosis and Gorlin syndrome.
Paller said she hopes that junctional EB will be the next candidate for gene-corrected grafts. However, she added, with more extensive clinical experience and cost reductions over time, grafting of gene-corrected skin could be considered to improve focal areas in other forms of EB and genetic skin disorders.
For the near term, Paller said she also hopes that insurers will not block access to the other approved RDEB treatments for patients who undergo prademagene zamikeracel treatment. “I trust that that won’t happen because these patients are so needy,” she said. To help patients access treatment, Abeona offers the Abeona Assist program, which helps patients understand their insurance benefits and financial assistance options and provides travel and logistical assistance.
“As far as I’m concerned,” said Paller, “each patient with EB should have everything at our disposal to help — this is such a horrible disease. If I can graft a 12 credit card-sized area and then keep them going with tricks for other areas, I’ll be very happy.”
The study was funded by Abeona Therapeutics, which developed prademagene zamikeracel, which also conducted data analysis and employs several study co-authors. Tang is listed on the prademagene zamikeracel patent, which is licensed by Stanford University to Abeona, but she receives no royalties. Additionally, Tang has consulted on EB-related therapeutics for BridgeBio and Fibroderm. Paller served on the VIITAL data safety monitoring board and has consulted for Chiesi, Krystal, and Castle Creek Biosciences.
John Jesitus is a Denver-based freelance medical writer and editor.
Source link : https://www.medscape.com/viewarticle/rdeb-genetically-corrected-skin-grafts-lead-sustained-2025a1000hse?src=rss
Author :
Publish date : 2025-07-04 07:09:00
Copyright for syndicated content belongs to the linked Source.