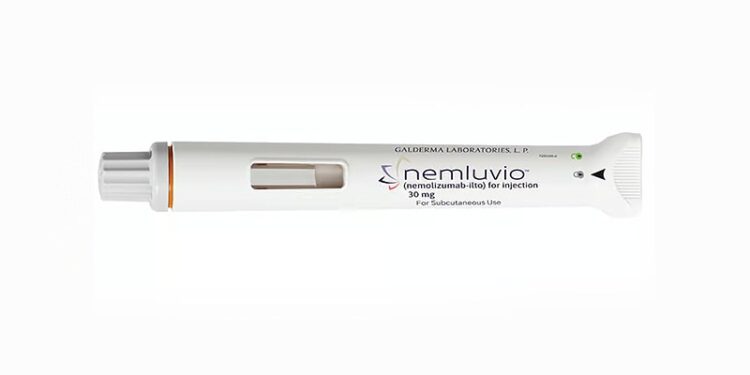
The Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for nemolizumab (Nemluvio, Galderma) to treat moderate to severe prurigo nodularis in adults and moderate to severe atopic dermatitis in adolescents and adults.
The approval covers prurigo nodularis in individuals aged 18 and older. It also includes atopic dermatitis, or eczema, in those aged 12 and over with a body weight of at least 30 kg.
Nemolizumab is approved for use alongside topical therapies when these alone are insufficient. The recommended dosage is 30 mg, administered via injection using a pre-filled pen or syringe.
The decision comes after an Access Consortium new active substance work-sharing initiative (NASWSI) procedure. The drug has also been approved in the European Union, the United States, and Switzerland for both conditions.
Julian Beach, the MHRA’s interim executive director of healthcare quality and access, said in a press release: “We’re assured that the appropriate regulatory standards for the approval of this medicine have been met. As with all products, we will keep its safety under close review.”
The National Institute for Health and Care Excellence is currently assessing whether to approve nemolizumab for NHS use in England. An appraisal committee is due to meet on 5 March and guidance is expected on 21 May 2025.
The Conditions
Prurigo nodularis is a chronic inflammatory skin condition characterised by firm, itchy bumps or ‘nodules’ that appear on the skin after it has been scratched for a long time due to pruritus, or itchiness. Patients may have a few or several hundred nodules, mostly appearing on the arms, legs, upper back, and abdomen.
The condition can interfere with sleep and psychological well-being. Estimates suggest that around 0.03% of the English population may be affected.
Atopic dermatitis, also known as atopic eczema, is a long-term condition characterised by red blotchy rashes and dry, itchy and inflamed skin. It affects one in five children and one in 10 adults in the UK.
Nemolizumab is a humanised IgG2 monoclonal antibody that inhibits interleukin-31 (IL-31) signalling. By selectively binding to IL-31, a cytokine involved in pruritus, inflammation, epidermal dysregulation, and fibrosis, the drug helps prevent the release of proinflammatory cytokines and chemokines.
Clinical Trials
The MHRA’s decision for prurigo nodularis was based on two phase 3 clinical trials involving 560 adults aged 18 years and older. Participants received nemolizumab monotherapy at 30 mg or 60 mg, depending on their baseline weight, or a matching placebo every 4 weeks for 16 or 24 weeks.
In both trials, around 60% of patients achieved a significant reduction in itchiness and around 30% achieved Investigator’s Global Assessment (IGA) success, defined as clear or almost clear skin.
Patients receiving nemolizumab were slightly more likely than controls to experience at least one adverse event, including headache or eczema. The drug’s safety and efficacy in patients under 18 years have not been established.
For atopic dermatitis, approval follows two double-blind, placebo-controlled phase 3 clinical trials involving 1728 adolescents and adults aged 12 and over. Participants were randomly assigned subcutaneous nemolizumab at 30 mg or a matching placebo once every 4 weeks alongside topical corticosteroids, with or without topical calcineurin inhibitors.
Across both trials, around 40% of patients achieved IGA success, with at least a 75% improvement in their Eczema Area and Severity Index (EASI) score from baseline.
Both drug regimens had similar safety profiles, with between 41%-50% of patients in both trials experiencing at least one treatment-emergent adverse event, the most common of which were hypersensitivity and injection site reactions.
Further information about nemolizumab will be published in the Summary of Product Characteristics and Patient Information leaflets. These will be available on the MHRA Products website within 7 days of approval.
Annie Lennon is a medical journalist. Her writing appears on Medscape.co.uk, Medical News Today, and Psych Central, among other outlets.
Source link : https://www.medscape.com/viewarticle/mhra-approves-nemolizumab-eczema-and-prurigo-nodularis-2025a100046n?src=rss
Author :
Publish date : 2025-02-18 15:32:29
Copyright for syndicated content belongs to the linked Source.