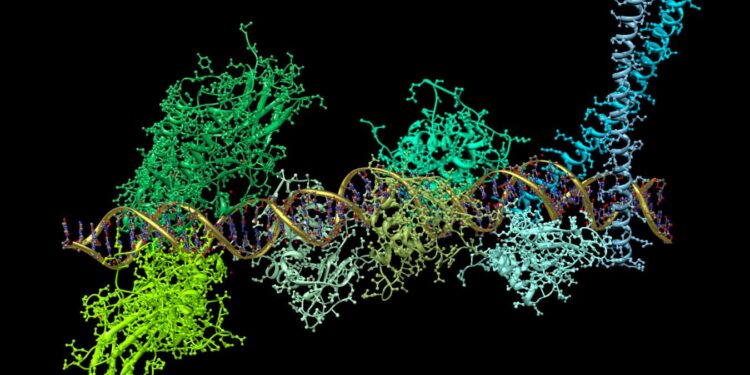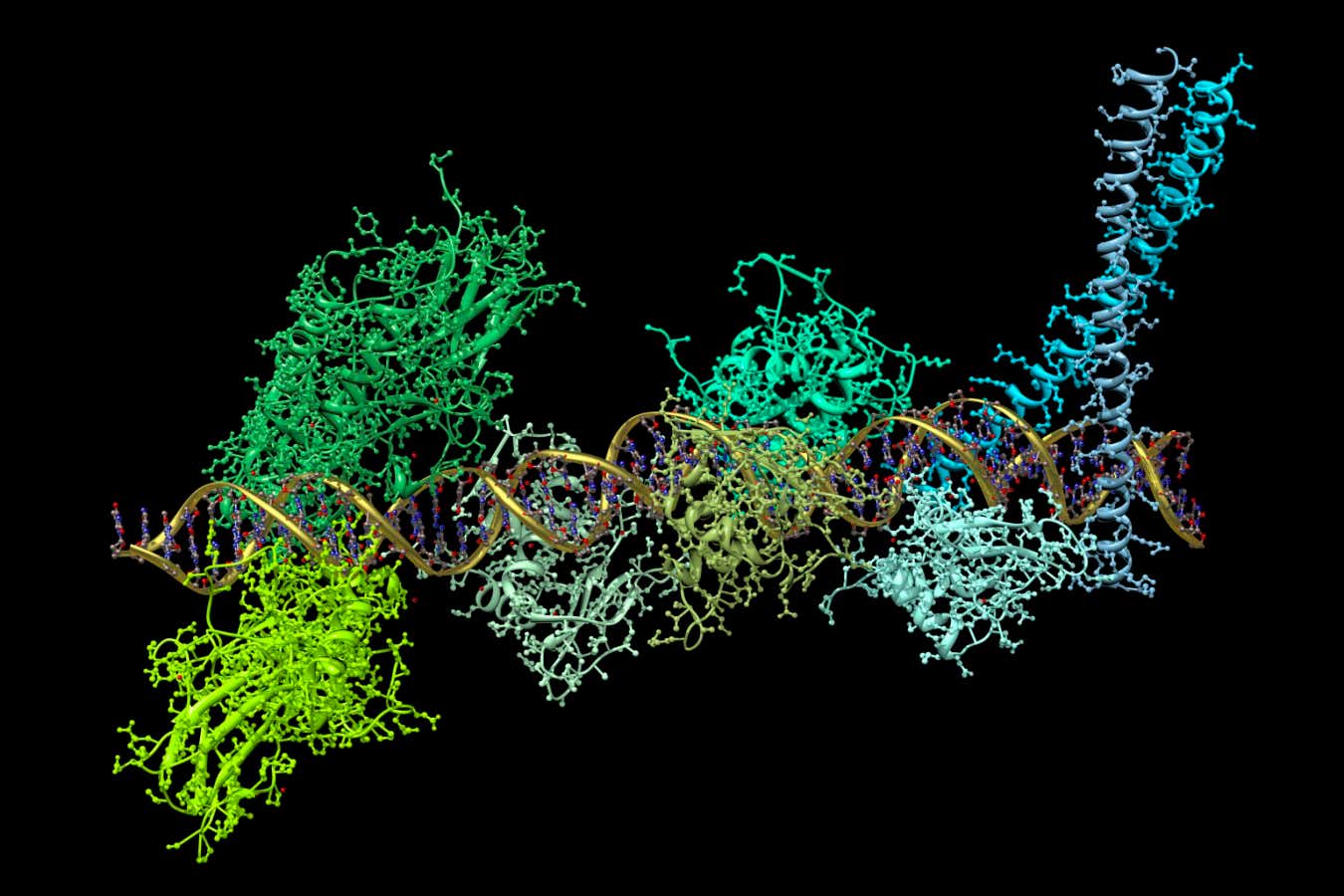
An illustration of a protein complex binding to DNA in the production of the key signalling molecule interferon
Martin McCarthy/Getty Images
Just one weekly puff from an asthma-like inhaler might one day protect you from the viral infections that make winters miserable – and could even save your life in the event of another pandemic.
That is the tantalising prospect raised by promising animal tests of an mRNA treatment that turns on our built-in viral defences. “You can think about this as a universal antiviral,” says Dusan Bogunovic at Columbia University in New York.
Realising the full promise of this approach will require further development of the mRNA technology used in vaccines – but last week the US slashed funding for mRNA vaccine development. “I would be surprised if it didn’t have knock-on effects on efforts like this,” says Bogunovic.
In addition to our body learning to recognise and target viruses with antibodies, it has lots of built-in defences. For instance, when a viral infection is detected, cells release a key signalling molecule called interferon. This turns on around 1000 genes, triggering the production of a wide array of antiviral proteins that work in many different ways: some block viral entry to cells, others inhibit the release of new viral particles.
Not all of these proteins work against all viruses, but in combination they can make a big difference. “Our innate immune system is extremely powerful,” says Bogunovic.
The problem is that viruses, especially respiratory ones, replicate really fast, says Bogunovic, so can outpace the body’s ability to ramp up its innate defences. But if the body gets a head start on preparing these defences, this can limit viral replication and keep infections mild, even before the rest of the immune system kicks in.
There were hopes that interferon could be used as a general antiviral, but it can have serious side effects. So Bogunovic and his colleagues are instead developing antivirals consisting of subsets of the 1000 proteins whose production is triggered by interferon.
They selected 10 of these proteins and delivered them to cells in the form of mRNAs coding for them. mRNA delivery means the proteins are temporarily produced inside cells where they are needed, whereas ready made proteins are too large to get inside cells in sufficient quantities.
Tests involving infecting human cells with a range of viruses, including flu and Zika, showed that this combination successfully boosted viral defences. In the body, this should provide a crucial head start.
Next, the team then delivered these mRNAs to the lungs of golden hamsters. The mRNA cocktail successfully protected the hamsters against the SARS-CoV-2 virus, which causes covid-19, with a dramatic reduction in viral numbers compared with untreated animals. “I was like, ‘wow, this actually might be a universal antiviral’,” says Bogunovic.
Existing antiviral drugs work only against specific viruses, so having a treatment that acts more broadly would be extremely valuable. The development of antibiotics like penicillin that can kill a broad range of bacteria revolutionised medicine.
What’s more, some combinations of interferon-triggered proteins might be especially effective against specific viruses, says Bogunovic. So the same approach could also be used to develop more specific antivirals.
Delivering the mRNAs to a high-enough proportion of the specific cells at risk of infection will be crucial. This is where further development is needed, as it is still difficult to deliver mRNAs to specific cell types.
“This is certainly exciting and could lead to very promising advances, but we are several steps down the line away from talking about a deployable, versatile countermeasure,” says Aris Katzourakis at the University of Oxford. “The research highlights the potential of mRNA technology beyond vaccines. The current trajectory in the US with mRNA vaccines will certainly and tragically slow down progress on both of these fronts.”
While antibiotic resistance is now a major problem, Bogunovic thinks viruses are unlikely to evolve resistance to this kind of antiviral as long as they include a range of interferon-triggered proteins targeting different aspects of the viral lifecycle. This combination approach has proved successful with HIV treatments, for instance.
Topics:
Source link : https://www.newscientist.com/article/2492205-mrna-drugs-could-protect-against-almost-any-kind-of-viral-infection/?utm_campaign=RSS%7CNSNS&utm_source=NSNS&utm_medium=RSS&utm_content=home
Author :
Publish date : 2025-08-13 19:00:00
Copyright for syndicated content belongs to the linked Source.