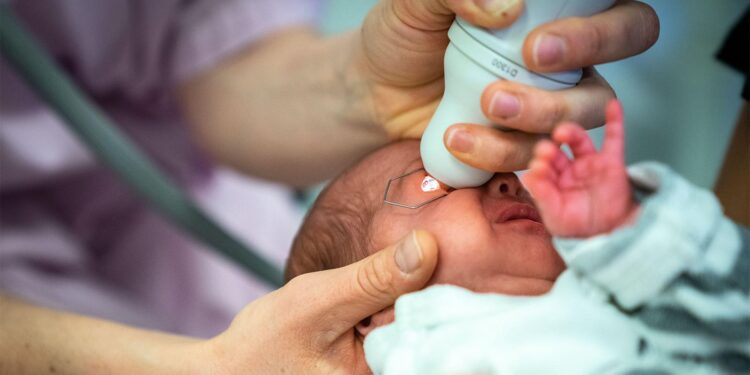
Mydriatic microdrops during retinopathy of prematurity (ROP) screening were noninferior to standard drops, which have been associated with cardiorespiratory and gastrointestinal adverse events, a randomized trial showed.
Among 83 preterm infants, microdrops were superior to standard drops for mydriatic efficacy — a measurement of dilation — at 45 minutes (mean difference 0.12, Bonferroni-corrected 95% CI 0.01-0.23, P=0.008) and noninferior at 90 minutes (Bonferroni-corrected 95% CI -0.10 to 0.17) and 120 minutes (Bonferroni-corrected 95% CI -0.18 to 0.14), reported Asimina Mataftsi, MD, PhD, of Aristotle University of Thessaloniki in Greece, and colleagues.
In addition, lower levels of oxygen saturation were observed after use of standard drops at 45 minutes (mean difference 0.66, 95% CI 0.09-1.24, P=0.03) and 90 minutes (mean difference 0.58, 95% CI 0.03-1.14, P=0.04), as was a higher percentage of 24-hour hypertensive episodes, with a median percentage of hypertensive episodes of 0.10% with microdrops compared with 0.14% with standard drops (P=0.01), they noted in JAMA Ophthalmology.
“This clinical trial provides evidence to support the use of microdrops in the vulnerable population of preterm infants,” the researchers concluded.
Infants at risk of ROP undergo several fundus examinations that require the use of eyedrops to cause eye dilation, Mataftsi and team noted. “These medications can have serious systemic adverse events, particularly cardiorespiratory and gastrointestinal, due to infants’ extremely low body mass and immature metabolizing capacities,” they wrote.
“In our study, despite the lower dose, microdrops seem to produce similar peripheral blood levels compared with standard drops within 3 hours’ post-instillation,” they pointed out.
A previous study linked eye examinations to higher numbers of apnea events (P=0.04) and noted that “there are many verbal reports from nurses working in NICUs of infants having adverse effects or generally deteriorating following the [ROP screening] procedure.”
Authors of a 2019 review also pointed out that “although the extent of mydriatic harm has not been fully investigated, a few studies signal the potential for cardiovascular, gastrointestinal, and respiratory adverse effects” from ROP screening. “It is very likely that our most vulnerable premature infants with pre-existing medical conditions are the ones who are more likely to experience adverse effects associated with mydriatics and are less likely to be able to compensate for any medicine-related harms,” they wrote.
For the MyMiROPS study, Mataftsi and colleagues included 83 preterm infants undergoing ROP screening at a tertiary center in Northern Greece from September 2021 to January 2023. To be eligible, infants had to have a gestational age below 32 weeks and/or a birth weight under 1,501 g, or be referred by an attending neonatologist due to comorbidities.
Mean gestational age was 29.7 weeks, 51% were boys, and mean birth weight was 1,277 g. Infants were randomized to three doses of microdrops (6.5 μL) or standard drops (28 to 34 μL) of a diluted 1.67% phenylephrine and 0.33% tropicamide mixture. Drops were administered at a random allocation sequence with a 1-week washout period.
The horizontal pupil diameter at 45, 90, and 120 minutes was measured using a customized ruler in 0.5-mm increments. The predefined noninferiority margin was -0.4 mm.
The researchers noted limitations to their study, including a lack of information about safety outcomes in 30% of patients.
“Future studies may … confirm our findings, extrapolate our results in different regimens and different populations, and provide more informative data about the overall systemic exposure,” they wrote.
Disclosures
This research received funding support from the European Paediatric Ophthalmological Society.
The study authors reported no conflicts of interest.
Primary Source
JAMA Ophthalmology
Source Reference: Seliniotaki AK, et al “Efficacy and safety of mydriatic microdrops for retinopathy of prematurity screening: the MyMiROPS randomized clinical trial” JAMA Ophthalmol 2024; DOI: 10.1001/jamaophthalmol.2024.5462.
Source link : https://www.medpagetoday.com/ophthalmology/generalophthalmology/113541
Author :
Publish date : 2024-12-26 16:00:00
Copyright for syndicated content belongs to the linked Source.

![author['full_name']](https://newshealth.biz/wp-content/uploads/2024/12/Mydriatic-Microdrops-May-Be-Better-for-Retinopathy-of-Prematurity-Screening.jpg)