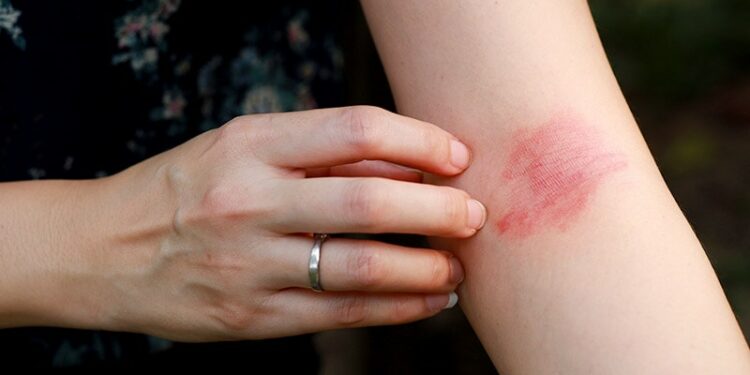
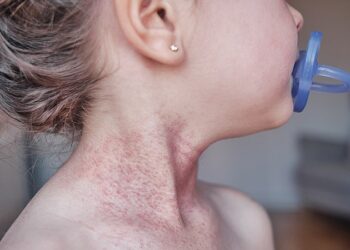
The US Food and Drug Administration (FDA) has approved nemolizumab for moderate-to-severe atopic dermatitis inadequately controlled with topical therapies in patients aged 12 years and older, according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin-31 (IL-31) receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, published in August in the Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75 compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
Source link : https://www.medscape.com/viewarticle/nemolizumab-gets-fda-nod-atopic-dermatitis-2024a1000n68?src=rss
Author :
Publish date : 2024-12-15 17:22:35
Copyright for syndicated content belongs to the linked Source.