The FDA approved ensartinib (Ensacove) as a first-line treatment for adults with ALK-positive locally advanced or metastatic non-small cell lung cancer (NSCLC), the agency announced on Wednesday.
Approval was based on results from eXALT3, a global open-label randomized trial that tested ensartinib against crizotinib (Xalkori) in 290 patients with locally advanced or metastatic ALK-positive NSCLC who had not previously received an ALK-targeted therapy.
Ensartinib doubled median progression-free survival (PFS) over the first-generation ALK inhibitor, with a median PFS of 25.8 months versus 12.7 months, respectively (HR 0.56, 95% CI 0.40-o.79, P=0.0007), meeting the study’s primary endpoint.
There was no statistically significant difference in overall survival (HR 0.88, 95% CI 0.63-1.23, P=0.4570).
Previously reported results from the trial also showed that ensartinib demonstrated superior efficacy against active brain lesions, with an intracranial response rate of 63.6% with ensartinib versus 21.1% with crizotinib for the subset with measurable brain metastases at baseline.
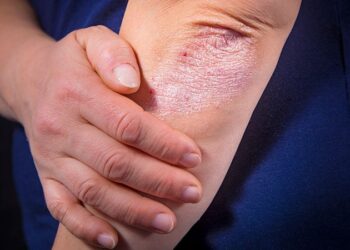
The most common adverse events (≥20%) with ensartinib included rash, musculoskeletal pain, constipation, cough, pruritus, nausea, edema, pyrexia, and fatigue.
Ensartinib joins a crowd of ALK inhibitors for NSCLC, with alectinib (Alecensa), brigatinib (Alunbrig), and lorlatinib (Lorbrena) all having previously topped crizotinib as well.
The recommended ensartinib dose is 225 mg orally once daily, with or without food, until disease progression or unacceptable toxicity.
Source link : https://www.medpagetoday.com/hematologyoncology/lungcancer/113462
Author :
Publish date : 2024-12-18 22:31:21
Copyright for syndicated content belongs to the linked Source.

![author['full_name']](https://newshealth.biz/wp-content/uploads/2024/12/New-First-Line-Option-for-Advanced-ALK-Positive-Lung-Cancer.jpg)