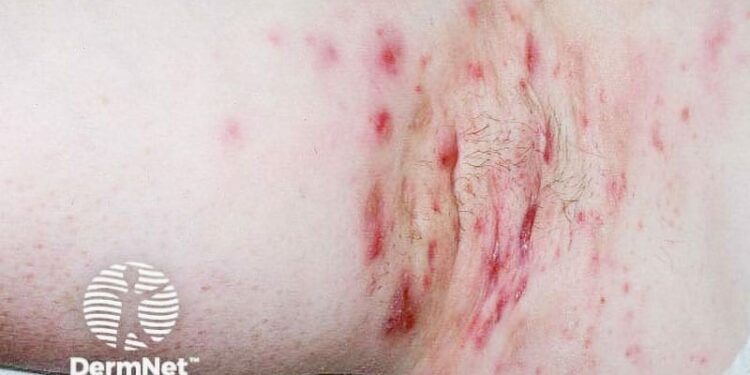
When it comes to managing hidradenitis suppurativa (HS) in special patient populations, new guidelines rate the existing evidence as “strong” for the following clinical scenarios: The continued use of biologics by pregnant patients throughout pregnancy, the use of adalimumab by breastfeeding patients who require biologics, and the use of oral doxycycline by patients with a history of malignancy who require systemic antibiotics for treatment of HS.
Those are just 3 of 119 consensus statements provided in the “North American Clinical Practice Guidelines for the Medical Management of HS in Special Patient Populations,” which were published online on December 24, 2024, in the Journal of the American Academy of Dermatology, a first-of-its-kind effort to establish recommendations for the medical management of HS in seven different scenarios: Pregnancy, breastfeeding, pediatrics, malignancy, tuberculosis (TB) infection, hepatitis B or C infection, and HIV disease.
“The need for clinical management guidelines for hidradenitis suppurativa in special populations has become especially urgent as new treatments for HS are emerging,” Haley Naik, MD, associate professor of dermatology at the University of California, San Francisco, who co-chaired a 14-member group of contributors that assembled the guidelines, told this news organization. “ While clinical trials provide essential data for FDA [Food and Drug Administration] approvals, they often exclude or underrepresent individuals with complex health conditions, such as those who are pregnant, breastfeeding, adolescent, or living with chronic infections or cancer.”
HS disproportionately affects women and marginalized groups, Naik continued, and prevalence is increased in patients with conditions like trisomy 21. The recognition of these gaps, coupled with the approval of new biologics, “made clear that clinicians need guidance on how to safely and effectively treat HS in these patient groups,” she said. “These guidelines are an essential step in improving care for these underserved patients.”
For the guidelines, Naik, project Co-chair Raed Alhusayen, MBBS, MSc, of the Division of Dermatology at Sunnybrook Research Institute, Toronto, Ontario, Canada, and coauthors, drew from the best available published evidence and expert consensus to establish recommendations for the medical management of HS in seven different patient populations. Next, they applied the Grading of Recommendations Assessment, Development and Evaluation approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant trials in medical literature.
Of the 27 recommendations made for patients with HS who are pregnant or who are planning a pregnancy, the authors ranked 8 as “strong” based on the evidence reviewed and the rest as “conditional.” The “strong” recommendations include the use of zinc supplements, avoidance of oral doxycycline because of the potential increased risk for congenital anomalies, avoidance of oral erythromycin because of an increased risk for elevated liver enzymes and other adverse outcomes, the use of metformin, continued use of biologic therapy, use of adalimumab in those who require biologics, and limiting the use of doxycycline among those who are breastfeeding.
Other categories of patients with HS include the following recommendations:
Breastfeeding: For those who require systemic antibiotics, a conditional recommendation is made for using oral rifampin. For those who require antiandrogens, a conditional recommendation is made for the use of metformin or the use of oral contraceptives. For patients who require systemic immunomodulators and biologics, a conditional recommendation is made for using prednisone
Pediatrics: For pediatric patients with HS who require topical treatment, a conditional recommendation is made for using concomitant antiseptic washes to decrease bacterial resistance. For adolescent females who require antiandrogens, a conditional recommendation is made for the use of spironolactone or the use of combined oral contraceptives. For pediatric patients who require biologics, a strong recommendation is made for using adalimumab in those 12 years of age or older.
Malignancy: For patients with a history of malignancy who require systemic antibiotics, a strong recommendation is made for using oral doxycycline. For those who require antiandrogens for HS, a strong recommendation is made for using metformin. For those who require systemic immunomodulators and biologics, a strong recommendation is made for the patient’s physician to consult with the patient’s oncologist to “take into account activity of HS, patient’s age, characteristics of the previous cancer (organ, stage, histological type, and prognosis), the time since completion of cancer treatment, and the individual carcinogenic effects of immunosuppressants,” the authors wrote.
TB infection: For those who require treatment for latent TB, a strong recommendation is made for using a 4-month course of oral rifampin. For those at high risk for active TB who require antiandrogens for HS, a strong recommendation is made for using metformin. For those who require glucocorticoids, a strong recommendation is made for annual screening for latent TB if the dose exceeds 15 mg of prednisone equivalent daily for at least 4 weeks.
Hepatitis B or C: For patients with hepatitis B or C who require systemic antibiotics for HS, a conditional recommendation is made for using oral ciprofloxacin, especially if there is evidence of cirrhosis. For those without cirrhosis who require antiandrogens for HS, a strong recommendation is made for using these agents “with an approach similar to other HS populations.” For patients with HS who require systemic immunomodulators and biologics, a strong recommendation is made to screen for hepatitis B and C prior to initiation of treatment.
HIV: For those with HS and HIV positivity who require systemic antibiotics, a conditional recommendation is made for using oral doxycycline. For those with HS and HIV positivity who require antiandrogens, a conditional recommendation is made for “considering non-metformin antiandrogens with an approach similar to other HS patient populations.” For patients with HS who require immunosuppressives or biologics, a strong recommendation is made for HIV screening before starting treatment.
Take-Home Messages
In Naik’s opinion, the guidelines highlight three key “take-home messages.” First, biologics can be safely used to treat HS during pregnancy and breastfeeding, ideally in close collaboration with obstetric and pediatric specialists. Second, biologic treatments can be safely and effectively administered to pediatric and adolescent patients with HS. Third, individuals with HS who also have concurrent chronic infections can safely undergo HS treatment with careful coordination with infectious disease and other specialists.
“These guidelines have reinforced the importance of formalizing coordinated multidisciplinary care for our most complex HS patients,” Naik said. “In my own practice, these guidelines have inspired even more regular and structured multispecialty discussions to ensure comprehensive treatment for each patient, which has improved care delivery.”
Robert Sidbury, MD, MPH, chief of the Division of Dermatology at Seattle Children’s Hospital, Seattle, who was asked to comment on the guidelines, characterized the effort as “extremely important” because of the significant challenges HS presents to clinicians, even under the best of circumstances. “Why? It is a devastating disease for which there are few effective treatment options, even fewer that are FDA approved, and often even these are denied by insurance carriers,” he said.
“In the special populations described by the authors, these challenges are amplified and can be difficult to overcome,” Sidbury added. “These guidelines provide evidence-based, easily accessible, structured support for providers in the clinic room and currency for our back-office staff to use in the fight against insurance denials.”
Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andMe, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera, is an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation. Alhusayen reported having received personal fees from AbbVie, Bausch Health, Boehringer Ingelheim, Fresenius Kabi, Janssen Pharmaceuticals, Novartis, Pfizer, Sandoz, and UCB; investigator fees from AbbVie, Bausch Health, Incyte, and Janssen Pharmaceuticals outside the submitted work. Other authors reported having financial disclosures with many pharmaceutical companies. Sidbury reported having no relevant disclosures.
Source link : https://www.medscape.com/viewarticle/new-hidradenitis-suppurativa-guidelines-focus-special-2025a10001fv?src=rss
Author :
Publish date : 2025-01-22 05:21:51
Copyright for syndicated content belongs to the linked Source.