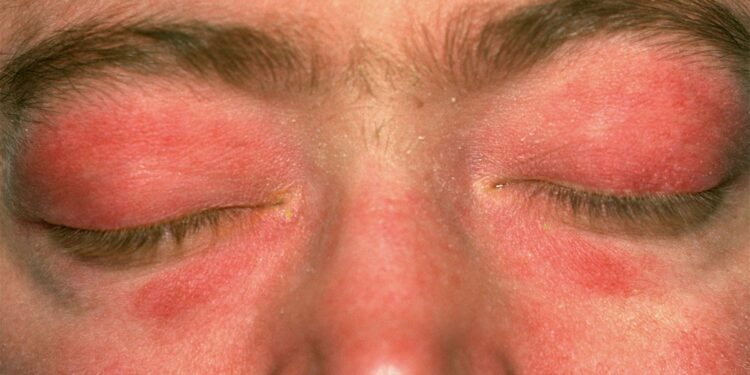
An investigational monoclonal antibody targeting interferon beta (IFNβ) led to rapid and pronounced reductions in disease activity in adults with dermatomyositis, a randomized phase II study showed.
In a skin-predominant cohort of the multistage study, those assigned to dazukibart 600 mg achieved a clinically meaningful and statistically significant decrease in week-12 scores on the Cutaneous Dermatomyositis Disease Area and Severity Index-Activity (CDASI-A; -18.8 vs -3.9 with placebo, P
And a pooled analysis of all patients with skin-predominant involvement across multiple stages of the trial showed a significant 12-week benefit with both tested doses of dazukibart versus placebo on the CDASI-A, a 100-point scale where higher scores indicate worse disease activity:
- 600 mg: placebo-adjusted difference -16.3 (90% CI -20.4 to -12.1, P
- 150 mg: placebo-adjusted difference -13.7 (90% CI -18.3 to -9.0, P
“This clinical trial is the first to show the effects of inhibiting IFNβ in patients with dermatomyositis, providing support for dazukibart as the first therapy developed to address the specific molecular pathology of dermatomyositis,” wrote Vleugels and co-authors in The Lancet.
The drug was well tolerated, and while a significant difference in CDASI-A score was not observed in the muscle-predominant cohort, “other secondary efficacy outcomes were positive with numerical improvements across all key muscle function outcomes” with the 600-mg dose of dazukibart, according to the researchers.
Dermatomyositis is a chronic systemic autoimmune disease characterized by cutaneous eruptions, muscle weakness, and systemic manifestations, including interstitial lung disease. The rare disease can negatively affect quality of life and is associated with substantial morbidity. The cause is unknown but may include genetic or environmental triggers.
Current treatments for dermatomyositis include various immunosuppressants and immunosuppressive agents, including corticosteroids, along with intravenous immunoglobulins (IVIG), but these options are not very effective and are associated with known side effects.
“These limitations underscore the urgent need to develop more specific and effective therapeutic strategies tailored to the unique pathophysiology of dermatomyositis,” noted Farida Benhadou, MD, PhD, and Elie Cogan, MD, PhD, both of the Université Libre de Bruxelles in Belgium, writing in an accompanying editorial.
“Advances in the early 2000s identified interferon beta (IFNβ) as a key contributor to the pathogenesis of dermatomyositis,” the editorialists explained. “Elevated IFNβ concentrations have been shown to be strongly associated with clinical indicators of disease activity in both skin and muscle. This observation highlights the role of IFNβ as a principal driver of inflammation in dermatomyositis.”
Dazukibart is a selective monoclonal antibody that targets IFNβ, blocking its receptor-mediated signalling. Prior phase I data showed an acceptable safety and tolerability profile, and a phase III trial is now underway for patients with active idiopathic inflammatory myopathies, including dermatomyositis or polymyositis.
The double-blind, placebo-controlled phase II trial from Vleugels and colleagues was conducted across 25 sites in Europe and the U.S. In total, 125 patients were screened and 75 patients were randomly assigned and treated with dazukibart, at a dose of 150 mg (n=15) or 600 mg (n=37), or placebo (n=23). Treatment was administered intravenously on day 1, and then every 4 weeks for up to 20 weeks depending on the stage of the trial.
Participants in the skin-predominant cohort were required to have a CDASI-A score of 14 or higher and to have failed on at least one systemic treatment; change from baseline to week 12 in CDASI-A was the primary outcome for this group. Exploratory analyses showed improvements on the disease activity scale were maintained even after stopping treatment.
Of the 57 participants in the skin-predominant cohort, nearly all were women (93%). Median age was 52 years, and 93% were white. Median CDASI-A score at baseline was 31, and the median duration of disease was 5 years. About one-fourth had received prior IVIG and nearly two-thirds had received prior corticosteroids, with about half on corticosteroids at study enrollment. Other medications at baseline included immunosuppressants/immunomodulators (84%), methotrexate (21%), mycophenolate mofetil (23%), and azathioprine (7%).
The researchers noted that all patients treated at the 150-mg dazukibart dose and 96% of those treated at the 600-mg dose had a clinically meaningful improvement of more than 5 points on the CDASI-A score.
“Furthermore, a decrease of more than 40% in CDASI-A score has been shown to indicate a meaningful change in quality of life for patients with a baseline score of more than 14 points,” wrote Vleugels and colleagues. “Importantly, dazukibart reached this threshold decrease in CDASI-A score in more than 80% of patients in our trial, all of whom had at least one unsuccessful standard of care systemic treatment.”
Those in the muscle-predominant cohort had to have active moderate muscle involvement, and safety was the primary endpoint for this group.
For the 18 patients in the muscle-predominant cohort, 72% were women, 89% were white, and the median age was 45 years. Median CDASI-A score at baseline was 13, while the median duration of disease was 2.2 years. Most (89%) had previously used corticosteroids and 44% had received IVIG. Other medications at baseline included corticosteroids (72%), immunosuppressants/immunomodulators (100%), methotrexate (44%), and mycophenolate mofetil (28%).
Safety analysis showed adverse events (AEs) in 80-81% of patients receiving dazukibart and 78% of those assigned to placebo — most were mild, and the most common AEs were infections and infestations.
Severe AEs occurred in four patients (11%) randomized to the dazukibart 150-mg group and one patient (4%) from the placebo group. Two patients discontinued dazukibart permanently.
Vleugels’ team noted that one patient in the muscle-predominant cohort who received dazukibart 600 mg followed by placebo “died during follow-up due to hemophagocytic lymphohistiocytosis and macrophage activation syndrome.” The AE was considered unlikely to be related to treatment or study conduct.
Disclosures
Pfizer funded the trial and multiple study authors are employed by the company.
Vleugels disclosed relationships with Argenx, Priovant, and Eli Lilly. Co-authors reported relationships with Pfizer, AbbVie, ActiGraph, Akari, Alexion, Amgen, ANI Pharmaceuticals, Argenx, AstraZeneca, Bayer, Beacon Bioscience, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Cabaletta Bio, Capella, Calyx, Celgene, Clarivate, Corbus, Corcept, Crisalis, CSL Behring, Cugene, Eli Lilly, Elorac, EMD Serono, Galapagos, Genentech, Gilead, GSK, Horizon, iCell, IGM Biosciences, Incyte, Janssen, Kezar, Kyowa, Kyowa Kirin, Kyverna, Mallinckrodt, Merck, miRagen, Momenta, Nektar, Novartis, Nuvig, Octapharma, Palvella, Phlecs, Priovant, Q32 Bio, Regeneron, Roivant, Rome Pharmaceuticals, Sanofi, Soligenix, Sun Pharma, Syntimmune, Teva, UCB, UpToDate, Viela, and Ventus, along with provisional and intellectual property patents related to skin conditions.
Benhadou and Cogan reported no competing interests.
Primary Source
The Lancet
Source Reference: Fiorentino D, et al “Efficacy, safety, and target engagement of dazukibart, an IFNβ specific monoclonal antibody, in adults with dermatomyositis: a multicentre, double-blind, randomised, placebo-controlled, phase 2 trial” Lancet 2025; DOI: 10.1016/S0140-6736(24)02071-3.
Secondary Source
The Lancet
Source Reference: Benhadou F, Cogan E “Targeting IFNβ in dermatomyositis” Lancet 2025; DOI: 10.1016/S0140-6736(24)02859-9.
Source link : https://www.medpagetoday.com/dermatology/generaldermatology/113791
Author :
Publish date : 2025-01-15 19:03:58
Copyright for syndicated content belongs to the linked Source.

![author['full_name']](https://newshealth.biz/wp-content/uploads/2025/01/Novel-Drug-Eases-Disease-Activity-in-Dermatomyositis.jpg)