TOPLINE:
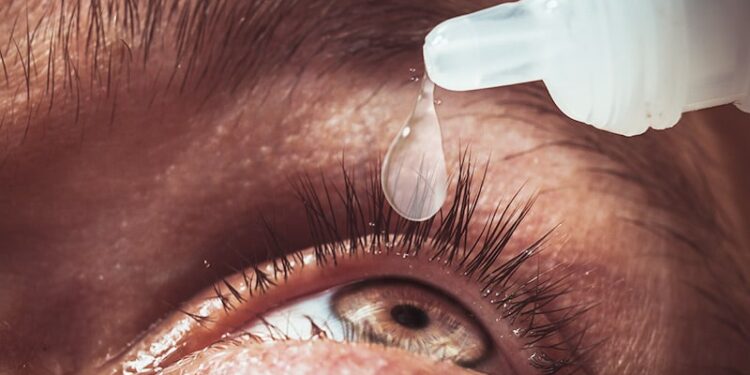
The daily use of eye drops containing 0.002% omidenepag isopropyl reduced intraocular pressure and was well‐tolerated in patients newly diagnosed with primary open‐angle glaucoma who had not previously received topical ophthalmic treatment.
METHODOLOGY:
- Researchers conducted a phase 4 clinical trial at four eye centers in Korea to evaluate the effectiveness and safety of the drops in patients newly diagnosed with primary open-angle glaucoma.
- Participants in the study had defects in their field of vision, had not previously received treatment for glaucoma, and had an intraocular pressure of 10-34 mm Hg; those with an intraocular pressure of ≤ 21 mm Hg were classified as having normal tension glaucoma.
- Participants (N = 50) received one drop of 0.002% omidenepag isopropyl ophthalmic solution each evening for 12 weeks; adherence to the medication was assessed at weeks 4 and 12.
- The primary endpoint of the trial was the change in intraocular pressure from baseline to week 12.
TAKEAWAY:
- Among the enrolled participants, the effectiveness of omidenepag isopropyl eye drops was evaluated in the 37 patients who achieved at least 70% adherence to the medication, while safety was assessed in all 50 participants.
- The mean intraocular pressure decreased significantly from 16.19 mm Hg at baseline to 13.55 mm Hg at week 12 (P < .0001), representing a 16% reduction; in the subgroup of patients with normal tension glaucoma, the mean intraocular pressure reduced from 15.79 mm Hg at baseline to 13.27 mm Hg at week 12.
- The medication effectively reduced the mean intraocular pressure as early as week 4 (P < .0001).
- The most common adverse events were hyperemia (13 cases) and iridocyclitis (5 cases), with no systemic reactions reported.
IN PRACTICE:
“Omidenepag isopropyl 0.002% ophthalmic solution had a rapid IOP-lowering effect as the percentage reduction from baseline was 15% at week 4,” the researchers wrote. “Omidenepag isopropyl 0.002% ophthalmic solution is suitable for first-line use at first diagnosis” of primary open-angle glaucoma, including in patients with normal tension glaucoma, they added.
SOURCE:
This study was led by Hyoung Won Bae, MD, PhD, of the Yonsei University Severance Hospital in Seoul, Korea. It was published online on June 17, 2025, in the Journal of Glaucoma.
LIMITATIONS:
This study lacked a control group and may have been subject to biases, as is common in observational studies. It also enrolled fewer participants than planned.
DISCLOSURES:
This study received funding from Santen Pharmaceutical Co., Ltd. All authors disclosed receiving honoraria from Santen.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/omidenepag-promising-against-glaucoma-real-world-study-2025a1000h15?src=rss
Author :
Publish date : 2025-06-25 11:20:00
Copyright for syndicated content belongs to the linked Source.