TOPLINE:
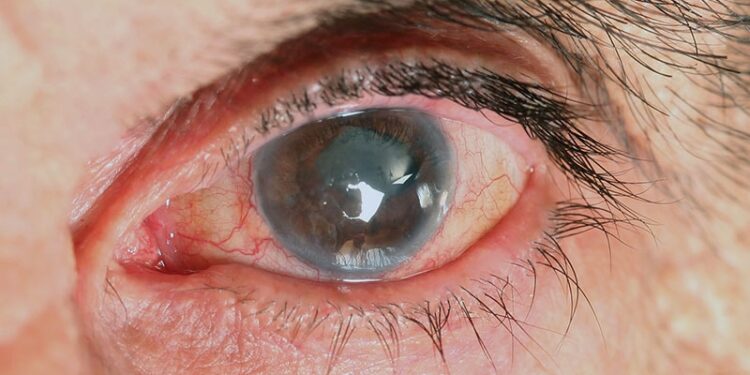
The use of bisphosphonates, particularly risedronate, in patients with osteoporosis is linked to a higher risk for acute angle closure but not open-angle glaucoma.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using a nationwide health claims database to assess whether the use of oral or intravenous bisphosphonates increased the risk for acute angle closure and open-angle glaucoma.
- A total of 208,111 patients with osteoporosis from the United States who used bisphosphonates or raloxifene (chosen as the reference drug) from 2010 to 2018 were included.
- Patients with acute angle closure and open-angle glaucoma were identified using diagnostic codes; patients without these diagnoses were designated as the control group.
- The analysis for acute angle closure included 372 patients with the diagnosis and 1488 patients in the control group (mean age, 70.4 years; mean follow-up, 2.9 years), and the analysis for open-angle glaucoma involved 3184 patients with the diagnosis and 12,736 patients in the control group (mean age, 71.5 years; mean follow-up, 2.7 years).
TAKEAWAY:
- Bisphosphonate users had a higher risk for acute angle closure than nonusers (adjusted incidence rate ratio [aIRR], 1.78; 95% CI, 1.05-3.01).
- Risedronate users were at a higher risk for developing acute angle closure than those using other bisphosphonates (aIRR, 2.12; 95% CI, 1.17-3.87).
- Patients who were prescribed risedronate more than a median of five prescriptions in the year before the event were at a higher risk for acute angle closure than those using other bisphosphonates (aIRR, 2.76; 95% CI, 1.11-6.90).
- No significant association was found between use of a bisphosphonate and open-angle glaucoma.
IN PRACTICE:
“The results from our study will have important public health implications as millions of patients around the world use bisphosphonates for osteoporosis treatment,” the authors of the study wrote. “Physicians prescribing bisphosphonates should discuss the risk of AAC [acute angle closure] with their patients when reviewing the side effect profile and also inquire on the use of bisphosphonates when evaluating patients with AAC,” they added.
SOURCE:
The study was led by Bonnie He, of the Department of Ophthalmology and Visual Sciences at Dalhousie University, Halifax, Nova Scotia, Canada. It was published online on December 27, 2024, in Eye.
LIMITATIONS:
The limitations of the study included lack of access to diagnostic information, such as intraocular pressure or visual field tests, which could have affected the assessment of glaucoma severity. The study also lacked power to assess different dosages of bisphosphonates and faced uncontrollable confounders, such as body mass index and race. The general practice database used may not have coded predisposing factors, such as hyperopia and myopia. The data were collected from a sample of the population, which may limit the generalizability of the findings to a broader population.
DISCLOSURES:
The study was supported by the Glaucoma Research Society of Canada Project Grant. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/bisphosphonate-use-linked-increased-risk-acute-angle-closure-2025a1000066?src=rss
Author :
Publish date : 2025-01-06 11:50:39
Copyright for syndicated content belongs to the linked Source.