TOPLINE:
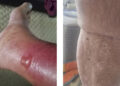
In a survey of 22 patients with hidradenitis suppurativa (HS), more than two thirds reported symptom improvement after treatment with glucagon-like peptide 1 receptor agonists (GLP-1 RAs).
METHODOLOGY:
- Researchers conducted a cross-sectional survey of 22 adults (average age, 45 years; 90.9% women) with HS who were treated with GLP-1 RAs at the University of Pennsylvania’s Dermatology Department between January 2019 and August 2024.
- Most participants were non-Hispanic (90.9%), 54.5% were Black, and 36.4% were White; 89.5% were classified as overweight or having obesity.
- GLP-1 RAs prescriptions were semaglutide (40.9%), tirzepatide (36.4%), dulaglutide (18.2%), or liraglutide (4.5%), with an average treatment duration of 17 months (range, 2-108 months).
- Primary outcomes were HS severity and quality of life.
TAKEAWAY:
- Most participants (77.3%) achieved weight loss averaging 31 lb; 68.2% reported improvement in HS-specific health, while 31.8% reported no change in their condition.
- Patient-reported symptom improvements included reduced flares (61.9%), new lesions (66.7%), pain (52.4%), drainage (61.9%), itch (47.6%), and odor (42.9%).
- Common side effects included gastrointestinal symptoms, allergic reactions, headaches, menstrual spotting, and appetite suppression.
- Nearly 60% of patients reported that HS had less impact on their daily activities, with the same percentage stating they would recommend GLP-1 RAs to other patients.
IN PRACTICE:
“These data suggest GLP-1 RAs may play an important adjunctive role in the treatment of HS, particularly given the high prevalence of obesity and diabetes in the HS population,” the study authors wrote. “Randomized controlled studies with robust patient and dermatologist-reported endpoints,” they added, “are needed to confirm these findings and establish clinical guidelines for use of GLP-1 RAs” in HS.
SOURCE:
The study was led by Radhika Gupta, BA, University of Pennsylvania, Philadelphia. It was published online on May 29 in JAAD International.
LIMITATIONS:
Study limitations included small size, lack of clinician assessment, high nonresponse rate, and potential recall bias.
DISCLOSURES:
The study received funding through a philanthropic gift for HS research. Gupta disclosed being a consultant for Cabaletta Bio. One author reported receiving research funding from Amgen, Boehringer Ingelheim, Cabaletta Bio, and InflaRx. Another author reported being a consultant for Sonoma Biotherapeutics, and the fourth author reported being a consultant for Vertex Pharmaceuticals.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/patients-hidradenitis-suppurativa-and-obesity-report-2025a1000f5e?src=rss
Author :
Publish date : 2025-06-05 04:19:00
Copyright for syndicated content belongs to the linked Source.