TOPLINE:
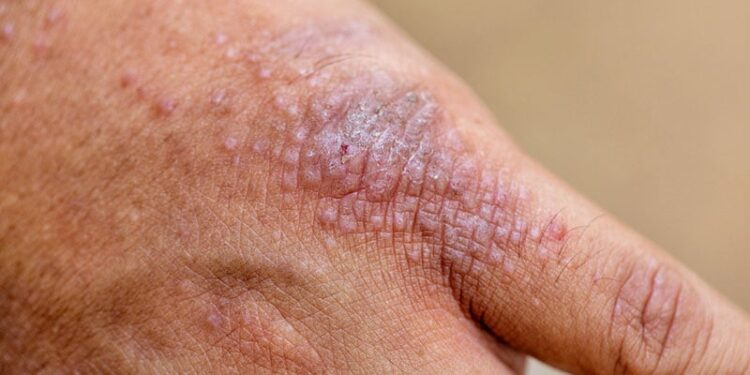
Upadacitinib was well tolerated in patients with moderate to severe atopic dermatitis (AD) and led to disease control as early as 1 month, with sustained improvements up to 12 months.
METHODOLOGY:
- Researchers conducted the UP-TAINED study to assess the effectiveness and safety of upadacitinib for over 2 years, with patients with moderate to severe AD receiving either 15 mg or 30 mg once daily.
- The coprimary endpoints were the proportion of patients achieving disease control (Atopic Dermatitis Control Tool total score < 7) at 3 months and the proportion of those who continued to maintain disease control through 24 months.
- Secondary endpoints included the proportion of patients achieving an Eczema Area and Severity Index (EASI) score ≤ 3, reductions of ≥ 75%, ≥ 90%, and 100% in EASI score, and skin clearance.
- This interim analysis reports the 1-year efficacy outcomes of upadacitinib in 351 patients with AD (mean age at baseline, 41.4 years).
TAKEAWAY:
- At 1 month, 68.8% of patients achieved disease control, which increased to 71.0% at 3 months and remained stable at 70.9% through 12 months.
- Overall, an early response was observed, with 60.6% of patients achieving an EASI score ≤ 3 after 1 month, increasing to 73.0% at 3 months.
- At 3 months, 77.2%, 60.5%, and 30.8% of patients achieved ≥ 75%, ≥ 90%, and 100% reductions in EASI scores, respectively, with similar response rates at 12 months.
- At 12 months, 76.7% of patients with moderate to severe hand eczema and 75.8% of patients with moderate to severe facial eczema achieved clear or almost clear skin.
- Treatment was well tolerated, with 426 adverse events (AEs) reported in 163 patients, most of which were mild (59.7%) or moderate (34.6%). The most common AEs were (worsening of) AD, acne, and COVID-19.
IN PRACTICE:
“The results of the UP-TAINED study demonstrated early and up to 1-year effectiveness of upadacitinib in patients with moderate to severe AD, especially in patients with AD in difficult-to-treat areas. Overall, upadacitinib was well tolerated in daily, real-world practice, and no new safety signals were identified,” the authors concluded.
SOURCE:
The study was led by Stephan Weidinger, Department of Dermatology and Allergy, University Hospital Schleswig-Holstein, Kiel, Germany. It was published online on March 15, 2025, in Dermatology and Therapy.
LIMITATIONS:
The interim analysis partially excluded dropout cases, potentially leading to an overestimation of efficacy outcomes. Additionally, not all patients had reached or had data available at the 1-year treatment mark, leading to smaller sample sizes. The study focused on the German population, possibly limiting generalisability to other populations.
DISCLOSURES:
The study was funded by AbbVie. Three authors reported being employees of AbbVie and owning stock in the company. Several authors reported receiving research grants or honoraria or having ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/real-world-study-reveals-early-success-upadacitinib-atopic-2025a10006fd?src=rss
Author :
Publish date : 2025-03-20 11:00:00
Copyright for syndicated content belongs to the linked Source.