TOPLINE:
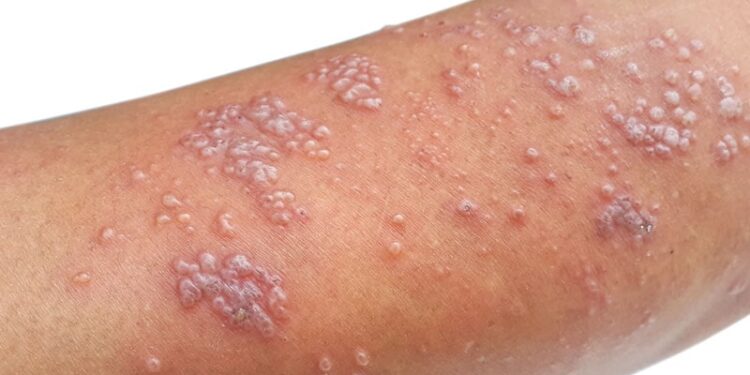
The recombinant zoster vaccine (RZV) Shingrix demonstrated effectiveness in preventing herpes zoster (HZ) and postherpetic neuralgia among adults aged 50 years or older with rheumatoid arthritis (RA). No increase in the risk for RA flares was observed within 30 days following vaccination.
METHODOLOGY:
- Researchers conducted a real-world study to assess vaccine effectiveness and safety of RZV (Shingrix) against HZ and postherpetic neuralgia in patients with RA aged 50 years or older.
- They enrolled 1926 individuals vaccinated with two doses of RZV and 5746 age- and sex-matched unvaccinated individuals (mean age, 69.4 years) from April 2018 to December 2021.
- Vaccine effectiveness of RZV against HZ and postherpetic neuralgia was estimated, overall and by age, after two doses received 4 weeks to 6 months apart or more than 6 months apart.
- Safety was assessed as the rate of RA flares within 30 days following RZV vaccination in 2606 individuals who received at least one dose of the vaccine as compared with the rate in self-controlled comparison periods.
- The average follow-up duration was 2.2 years for individuals vaccinated with two doses and 1.8 years for those who remained unvaccinated.
TAKEAWAY:
- The overall adjusted vaccine effectiveness of two doses of RZV against HZ and postherpetic neuralgia was 60.7% (95% CI, 41.0%-73.8%) and 88.7% (95% CI, 12.1%-98.5%), respectively.
- The vaccine effectiveness of two doses of RZV given 4 weeks to 6 months apart against HZ was 57.9% (95% CI, 34.4%-73.0%) and 73.5% (95% CI, 21.2%-91.1%) when given more than 6 months apart.
- No increased risk for RA flares was observed within 30 days postvaccination (relative risk, 1.02; 95% CI, 0.75-1.37).
IN PRACTICE:
“This study supports the benefits of RZV vaccination for preventing HZ in patients who have RA to reduce the HZ burden in this population,” the authors wrote.
SOURCE:
This study was led by Emily Rayens, PhD, MPH, Kaiser Permanente Southern California in Pasadena, California. It was published online on March 7, 2025, in Annals of the Rheumatic Diseases.
LIMITATIONS:
Results of this study may not apply to patients receiving care in different healthcare systems, uninsured populations, or other countries. The follow-up time after the index date was limited. Additionally, vaccine effectiveness associated with a single dose of RZV was not reported in this interim analysis due to the small sample size.
DISCLOSURES:
This study was supported by funds from GSK, which makes Shingrix. Four authors were employed at GSK during the study and held financial equities in the company. Other authors disclosed receiving funding for studies unrelated to this one from Dynavax, Gilead Sciences, Moderna, Genentech, VoxelCloud, Johnson & Johnson, and/or Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/recombinant-zoster-vaccine-proved-effective-patients-2025a10006o3?src=rss
Author :
Publish date : 2025-03-20 11:40:00
Copyright for syndicated content belongs to the linked Source.